<< Hide Menu
3.9 Separation of Solutions and Mixtures Chromatography
6 min read•june 18, 2024
Dalia Savy
Kanya Shah
Dalia Savy
Kanya Shah
When dealing with solutions, you will have a solute (or multiple solutes) dissolved in a solvent. Many times, a chemist will need to separate these solutions, especially after a chemical reaction has taken place. You might even have to go through some of these separation techniques in the lab! 🧪
Separating Solutions into Solute and Solvent
There are many methods to separate solutes from their solvent based on physical properties and differences in their intermolecular forces.
Evaporation
Evaporation is a process by which a solvent is boiled and thus evaporated in order to separate out the solute. For example, if you boil salt water🌊 long enough, you will be left with a small amount of salt🧂 at the bottom of your pot.
The same idea applies to essentially any solution with a solid, soluble solute. Evaporation is the simplest kind of separation and is often the one most students have the most experience with and understand the easiest. Essentially, just boil the liquid away!

Image Courtesy of eschooltoday.com
Filtration
Filtration is also a simple one to understand. When filtering a mixture, one runs the mixture through a porous barrier. With this barrier, the liquid passes through, but the solid gets trapped in the filter.
☕ A good real-life example of filtration is when making coffee, you pour the mixture of coffee beans and hot water through a coffee filter to let the coffee run through into your mug, but the ground coffee beans stay behind.
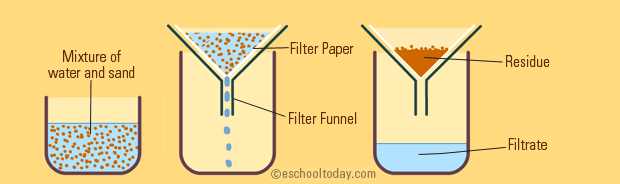
Image Courtesy of eschooltoday.com
Imagine you are filtering a solution of salt, water, and sand. Can you guess what will get caught up in the filter paper? ONLY the sand will. This is because the salt will dissolve in the water since it is not insoluble.
Only insoluble substances will be filtered with filtration. The filtrate would then be both salt and water, or salt water. Therefore, filtration isn't effective if you want to fully separate all of these elements. After filtering the sand out, you would have to evaporate the salt water solution to separate the salt.
Chromatography
Chromatography is a technique that separates chemical species based on their interaction with a stationary phase, which is typically a solid surface or a liquid. There are several types of chromatography, including paper chromatography, thin-layer chromatography, and column chromatography. Each of these techniques has its own specific set of advantages and disadvantages, and they are all used for different purposes depending on the characteristics of the sample being analyzed.
Paper Chromatography
Paper chromatography is a simple and cost-effective technique that is commonly used to separate and identify compounds in a sample. In this technique, a small amount of the sample is applied to a strip of chromatography paper, which is then placed in a solvent. The solvent moves up the paper by capillary action, and the compounds in the sample are carried along with it. As the solvent moves up the paper, the compounds in the sample interact differently with the paper, resulting in different rates of movement. This allows the compounds to be separated based on their affinity for the paper.
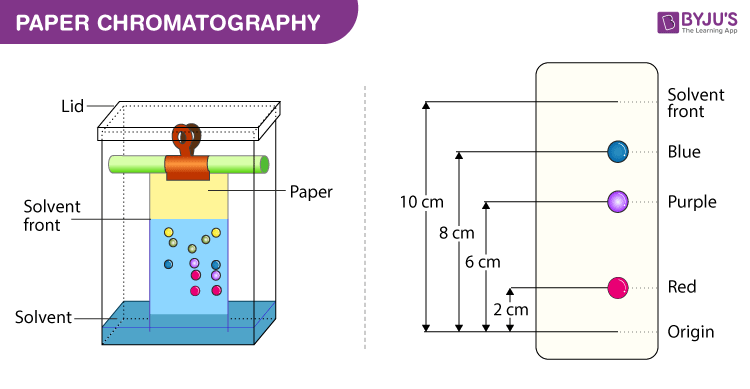
Image Courtesy of Byju's
Think of the solvent traveling up the paper and essentially "dragging" each part of the solution away from itself via differences in intermolecular forces and polarity.
The stationary phase in paper chromatography is typically a cellulose material, which is polar in nature. As a result, polar compounds in the sample will have a greater affinity for the cellulose paper, while non-polar compounds will have a weaker affinity. This leads to the separation of the compounds based on their polarity, with polar compounds moving more slowly up the paper and non-polar compounds moving more quickly.
** Remember: Like dissolves like with polarity! **
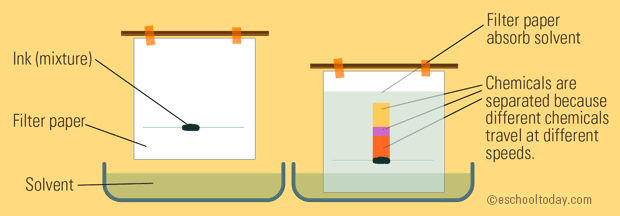
Image Courtesy of eschooltoday.com
Thin-Layer Chromatography (TLC)
Thin-layer chromatography is a technique that separates compounds in a sample using a stationary phase that is coated onto a thin layer of glass, plastic, or aluminum foil. Like paper chromatography, TLC relies on the differential affinity of compounds for the stationary phase to separate them (based on polarity). However, TLC has several advantages over paper chromatography, including faster separation times, the ability to use multiple solvents, and the ability to visualize the separated compounds using a variety of techniques.
Most TLC plates are made up of polar silica, which you can think of as a very small powder. This silica is considered the stationary phase, while the solvent in the TLC chamber is considered the mobile phase. A general rule of thumb is that polar compounds are more strongly attracted to the stationary phase, and will move less, while nonpolar compounds are more readily eluted with the solvent. This is very similar to paper chromatography, but here, the stationary phase is silica, not a cellulose material.
Column Chromatography
Column chromatography is less tested on the AP compared to TLC and paper chromatography, but it is still included on the AP Chemistry Course and Exam Description provided by College Board!
It is a technique that separates chemical species based on their interaction with a stationary phase, like the other types of chromatography. The difference is that the stationary phase is packed into a column, hence its name!
With column chromatography, the stationary phase is typically silica or alumina, which are both small sold materials. As the compounds pass through the column, they interact with the stationary phase, which results in different rates of movement. This, again, allows the compounds to be separated based on their affinity for the stationary phase.
This technique is more complex and takes more time than the other two, but it allows for the separation of large quantities of a sample and can achieve higher purity of separated components.
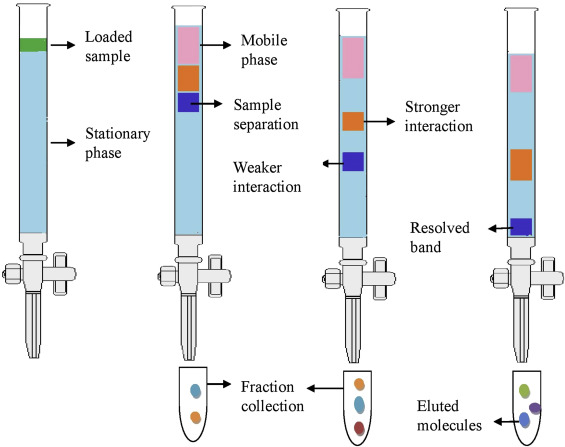
Image Courtesy of Science Direct
🥬 Fun fact, you can separate the different pigments in spinach with this technique!
Distillation
Distillation, like chromatography, is used to separate solutions of liquids. With distillation, the fact that different liquids have different boiling points is taken advantage of. Through distillation, a solution is carefully heated such that only one liquid boils out and then recondenses into another beaker. The first liquid to boil is the one with the weakest IMFs and the strength of IMFs increases as the boiling point increases.
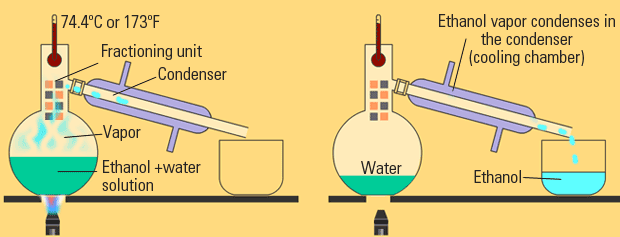
Image Courtesy of eschooltoday.com
There are several types of distillation, but let's discuss the differences between fractional distillation and simple distillation:
- 🌡️ Difference in boiling points: Simple distillation is typically used to separate a mixture of compounds that have a large difference in their boiling points. Fractional distillation is used to separate a mixture of compounds that have a smaller difference in their boiling points, or when the separation of the compounds is not possible using simple distillation.
- 💦 Number of vaporization and condensation steps: Simple distillation involves only one vaporization and condensation step, while fractional distillation involves multiple vaporization and condensation steps. This allows for a more refined separation of the compounds in the mixture.
- 💎 Purity of the separated compounds: Fractional distillation generally results in a higher purity of the separated compounds compared to simple distillation, due to the additional vaporization and condensation steps. 👉 Read this study guide to go through an AP Chemistry free-response question about paper chromatography!

© 2024 Fiveable Inc. All rights reserved.