<< Hide Menu
Dalia Savy
Kanya Shah
Dalia Savy
Kanya Shah
Remember when we went over matter in unit one? Matter is any physical object that has mass and occupies space. Matter can be classified according to its state (which we'll go over in this study guide), or its composition (the atoms or molecules that make it up).
Gases and solutions are big focuses of this unit, so understanding the content we're going to go over is essential!
Let’s look over some of the characteristic properties of the states of matter:
🧊Solids
As we learned in the last key topic, solids can either be crystalline or amorphous. Crystalline solids have a structure and 3-D order, while amorphous solids have considerable disorder within their structures.
Regardless of the type of solid, all solids retain their own shape and volume. They don’t expand to fill their container because the particles in a solid are packed very closely together and cannot move. The intermolecular forces are strong enough to keep the particles in place.
Although particles in a solid cannot move, they vibrate back and forth!
In general, solids are virtually incompressible, don’t flow, and the diffusion process within a solid occurs extremely slowly. Compressibility is the degree to which a material can be squeezed, or a measure of volume change when pressure is applied to a substance.
💧Liquids
Liquids assume the shape of a portion of the container it occupies. Liquids do NOT expand to fill their container, they just fill the space that is provided by the container.
Since liquid particles aren't tightly packed together, they have the ability to flow past one another (fluidity). The intermolecular forces are strong enough to hold the molecules closely together, but not strong enough to hold them in place.
Liquids are virtually incompressible like solids, flow readily, and diffusion within a liquid occurs slowly.
** The solid and liquid phases of a particular substance typically have similar molar masses since the particles are closely held together.**
Surface Tension
Surface tension refers to the tendency of liquids to minimize their surface area. It results from an imbalance of intermolecular forces when molecules on a surface of a liquid experience a net inward force.
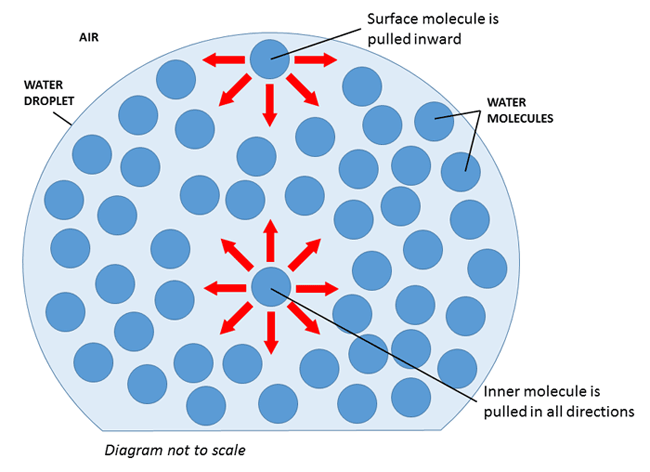
Image Courtesy of Pinterest
The interior, or bulk, molecules are attracted to particles in all directions, while surface molecules only experience attraction in lateral and downward directions. This causes surface molecules to be less stable, so liquids attempt to reduce the number of surface molecules they have. They do this by minimizing their surface area! You see this happening all the time in real-time:
Image Courtesy of Gizmodo
Whenever you turn the sink on at low pressure, the particles form a spherical shape to reduce their surface area.
There are two trends with surface tension that you should note:
- The stronger the intermolecular forces experienced, the higher the surface tension. This is because the surface molecules resist penetration, which increases surface area.
- The higher the temperature, the lower the surface tension. This goes into unit five and the movement of particles, but the higher the temperature, the easier it is to stretch the surface of particles.
Capillary Action
Capillary action is the spontaneous rising of a liquid against gravity. It often happens with polar liquids that have strong IMFs. You could see this when you put a paper towel🧻 in contact with a puddle; the water will slowly rise up the towel due to capillary action.
There are two different types of forces involved in capillary action:
- Cohesive Forces: forces between the liquid molecules that hold the liquid together
- Adhesive Forces: forces between liquid molecules and the container Capillary action occurs because the adhesive forces pull the surface molecules up and the cohesive forces pull the bulk molecules up with it. These two forces work together for it to occur!
On the other hand, the meniscus is due to the competition between these two forces.
Remember the typical meniscus you see when you do experiments? The meniscus of water is concave because the adhesive forces are stronger than the cohesive forces, so the water is more strongly attracted to the graduated cylinder than itself🧪.

Image Courtesy of Bartleby
I bet you've never seen Mercury in a glass container! Well, that creates an upside-down meniscus, which is called a convex meniscus. This occurs because the forces between the mercury molecules are stronger than the forces between the molecules and the glass (cohesive > adhesive).
Viscosity
Viscosity is a measure of a liquid's resistance to flow. You see this with syrup every time you eat pancakes🥞.
The stronger the intermolecular forces present, the higher the viscosity, and the thicker the liquid. The higher the temperature of the liquid, the lower its viscosity. This, again, goes back to unit five, but think of temperature as motion and an increased ability to overcome intermolecular forces.
♨️Gases
Gases assume the volume and shape of their container. Gas particles move rapidly in straight lines; more about the behavior of gases is covered in the rest of this unit!
The molecules have enough energy to overcome any intermolecular forces that exist, allowing them to move freely. They are compressible, flow readily, and expand to fill the container. Diffusion within a gas occurs quite rapidly.

Image Courtesy of the Schools of King Edward VI in Birmingham
Density
Density measures how compact a substance is! The formula for Density is D = m/V or density equals mass divided by volume.
It is good to remember that solids are usually the most dense out of the three phases and gases are usually the least dense since they flow freely in open space.
Density Practice Question
A student measured the mass of a sealed 644 mL flash that contained air. The student then flushed the flask with an unknown gas, resealed it, then measured the mass again. The air and the unknown gas were at STP. Calculate the mass of the unknown gas. The density of air at STP is 1.29 g/L.
| Volume of sealed flash | 644 mL |
| Mass of Sealed flask and air | 121.03 g |
| Mass of Sealed Flask and Unknown Gas | 122.60 g |
Before answering the question, let's note that the air is in a sealed flask. This means that it cannot escape into the atmosphere so the most it could do is fill the shape of the container.
Also, don't worry about STP yet, we'll go over that in the next key topic.
First Step - Find the mass of the air: Since we are given both the density of the air and the volume of the sealed flask, we can find the mass. However, they give us a density in g/L and a volume in mL, so first we have to convert 644 mL into 0.644 L by simply moving the decimal 3 to the left. D = M/V --> 1.29 = M/.644 --> M = 0.831 g
Second Step - Find the mass of the flask: Since we now know the mass of the air and the mass of both the air and the flask, we can just subtract: 121.03 g - 0.831 g = 120.20 g
Final Step - Find the mass of the unknown gas: They have given us the mass of both the sealed flask and the unknown gas. Since we know the mass of the sealed flask alone, we can just subtract and get our final answer! 122.60 g - 120.20 g = 2.40 g
Overview
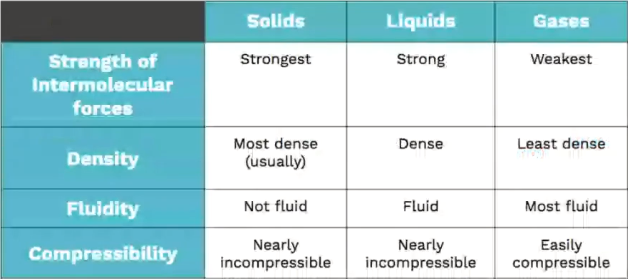
To remember the differences between a solid, liquid, and gas, think about your own experiences in life. For example, liquids, like water, will flow freely whereas a solid, like ice, maintains its shape no matter what container it is in. Here's a chart from our states of matter live stream to help out:

© 2024 Fiveable Inc. All rights reserved.