<< Hide Menu
1.8 Valence Electrons and Ionic Compounds
8 min read•june 18, 2024
Dalia Savy
Dalia Savy
We've discussed valence electrons several times in this unit's study guides already! Here is a quick recap regarding what you should know so far:
- Valence electrons are the outermost electrons in an atom.
- Valence electrons are found in the s and p orbital of the outermost shell.
- A gap in ionization energies could tell us how many valence electrons an element has.
- Elements in the same group on the periodic table have the same number of valence electrons.
Foundational Knowledge of Bonding
Valence Electrons
From the periodic table, you could tell how many valence electrons an element has.
Image Courtesy of ck12
The graph above skips over the transition metals because those are much less predictable. The AP Chemistry exam is going to primarily focus on the other elements, but it is good to be familiar with several transition metals (like Co, Cu, Zn).
Just by looking at the periodic table, you could tell that oxygen has six valence electrons and carbon has four valence electrons. Remember that the number of valence electrons an atom has affects the way the element bonds with others. Therefore, elements in the same group tend to bond with similar elements and form similar compounds. Here are some examples:
- Elements in group one can all bond with chlorine: LiCl, NaCl, KCl, RbCl.
- Elements in group two can all bond with O: MgO, CaO, SrO, BaO.
Charges of Ions
Before getting into bonds, it is also good to memorize the charges of most elements on the periodic table when they bond with another element. This will make more sense once we look into what happens when elements form a bond.
Essentially, ions are charged atoms or molecules that have gained or lost electrons. Atoms may become ions in order to achieve a more stable electron configuration. Remember, most things in chemistry try to become more stable! ⚖️
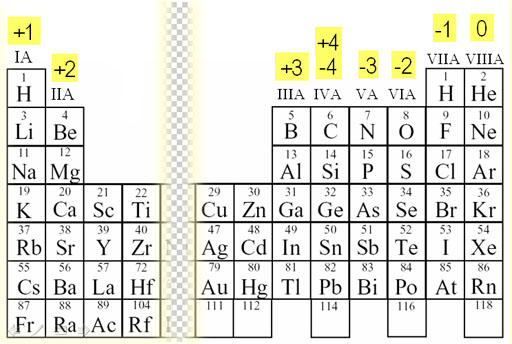
Image Courtesy of Chemistry Land
Again, the transition metals aren't included here because they have various charges and very different properties. Don't worry though, the AP won't question you about them. At most, you would have to write their electron configurations.
Types of Elements
There are three different types of elements: metals, nonmetals, and metalloids.
Image Courtesy of ck12
Metals are good conductors of heat and electricity🔥⚡, shiny, malleable (can bend), and ductile (can be made into a wire).
Nonmetals are the complete opposite: bad conductors of heat and electricity and brittle.
Metalloids have properties of both metals and nonmetals and there are only seven of them.
Electronegativity
Electronegativity refers to how strongly a nucleus attracts electrons of another atom. This comes into play when two atoms are sharing valence electrons since the pull of the electrons depends on how electronegative the atom is!
This is one of the five essential periodic trends to know and understand for AP Chemistry. Remember that fluorine is the most electronegative element on the periodic table, with a value of 4.0. From here, you can try to compare other elements to where fluorine is located on the periodic table.
👉 Want a quick refresh on electronegativity? Check out our study guide on "Periodic Trends."
Types of Bonds
Elements bond to achieve the lowest possible energy to reach the highest stability. There are two different types of bonds you should be familiar with: ionic bonds and covalent bonds.
Ionic Bonds
Ionic bonds are formed by the transfer of electrons from one atom to another, usually from a metal to a nonmetal.
The atom that loses an electron will gain a positive charge and is called a cation (usually a metal).
The atom that gains an electron will gain a negative charge and is called an anion (usually a nonmetal).
Some properties of ionic compounds include very strong bonds, solubility in water, and the ability to strongly conduct heat and electricity.
Example of an Ionic Bond - NaCl
In the ionic compound NaCl, sodium (Na) loses an electron and obtains a positive charge, while chlorine (Cl) gains an electron and therefore obtains a negative charge.
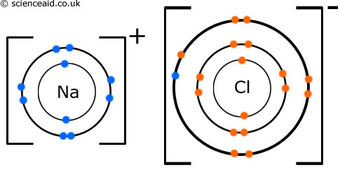
The one valence electron that was in Na was transferred to the chlorine atom in order for both ions to have a full octet. The octet rule is based on the idea that atoms are most stable when they have eight valence electrons in their valence shell.
Group one elements and group 17 elements (halogens) often bond this way to reach stability. They have the proper number of valence electrons that enable them to both reach a full octet. In fact, when forming an ionic bond with halogens, group one elements lose an entire electron shell.
When sodium and chlorine become ions, their electron configuration actually matches that of the noble gas closest to it. Here is an example:
Covalent Bonds
Covalent bonds are formed when atoms share electrons (usually two nonmetals). Some properties of covalent bonds include low melting points and weak electroconductivity abilities.
There are actually two types of covalent bonds: polar covalent bonds and nonpolar covalent bonds.
- Polar covalent bonds are a type of bonding where electrons are unequally shared between two different nonmetals. This is due to the two atoms having significantly different electronegativities.
- Nonpolar covalent bonds are a type of bonding where electrons are equally shared between, usually, two of the same nonmetal. This bond typically forms between two atoms that have a similar tendency to attract electrons, or rather, two atoms that have very similar electronegativities. Covalent bonds are strong and stable, and they are actually responsible for the properties of many common substances that we interact with on a daily basis. Some examples include water, methane, carbon dioxide, and even proteins, DNA, and carbohydrates! 🤯
Polar-Covalent Example - HF
Hydrogen and fluorine create a polar covalent bond. Fluorine attracts electrons more strongly due to its high electronegativity of 4.0, resulting in an unequal distribution of electrons. Think about it this way: fluorine is super greedy to fulfill its full octet of eight valence electrons, so it more strongly attracts them towards it.
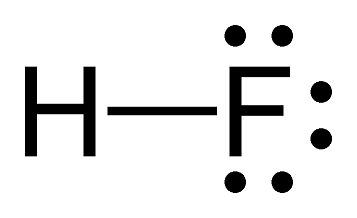
Image Courtesy of Study
Note the difference in representation between a covalent bond and an ionic bond. The dash here represents two shared electrons. Rather than a transfer of electrons, hydrogen is sharing one electron and fluorine is sharing one electron to enable both atoms to have their full octet.
Nonpolar Example - Cl2
In the covalent compound Cl2, two chlorine atoms bond together to share the final electron that they need to have their full octet.
Image courtesy of Wayne Breslyn
Just like in a polar covalent bond, they are sharing electrons with each other to obtain the full octet. However, note here that two atoms of the same element are bonding. In other words, their electronegativities are the same, so they would have the same pull on the electrons they share.
This does not mean that all nonpolar covalent bonds are between two atoms of the same nonmetal. The carbon-oxygen bonds in carbon dioxide (CO2), as well as the carbon-hydrogen bonds in methane (CH4) are also nonpolar covalent. Take a look at their electronegativities:
- Electronegativity of hydrogen: 2.2
- Electronegativity of carbon: 2.55
- Electronegativity of oxygen: 3.44
Charges and Partial Charges
When two atoms interact with each other in a bond, there is often a distribution of charge. Let's take a look at the different charge distributions we can see.
In a nonpolar covalent bond, the electronegativities of the two atoms are very similar, if not the same. Therefore, the electrons in the bond will be shared equally, resulting in a neutral charge distribution. The positive charge of the two nuclei is balanced by the negative charge of the shared electrons.
When there is a large gap in electronegativities between the two atoms in a bond, we start to see charges. In a polar covalent bond, two atoms, such as fluorine and hydrogen, share electrons unevenly. Fluorine has an electronegativity of 4.0, while hydrogen has that of 2.2. The atom with the higher electronegativity (F) will have a partial negative charge, while the atom with the lower electronegativity (H) will have a partial positive charge. This is because the very negative electrons are shifted towards the fluorine, creating an abundance of negative charge, and away from the hydrogen, creating a "loss" of positive charge.
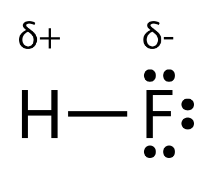
Image Courtesy of Chem LibreTexts
The Greek letter delta (δ) is used to indicate an atom that has either a positive or negative partial charge.
In an ionic bond, the transfer of electrons forms ions with complete opposite charges. Take a look at the transfer of electrons between the sodium and chlorine atoms above.
The loss of an electron causes the sodium to be a little extra positive (hence its +1 charge), whereas the gain of an electron causes the chlorine to be a little extra negative (hence its -1 charge). The reason why metals are typically the atoms to donate an electron is that they have very low electronegativities. Sodium has an electronegativity of 0.93, while chlorine has an electronegativity of 3.16. Compare this difference to that between hydrogen and fluorine! This is why the charges are not partial.
** Atoms with low electronegativities are more likely to donate electrons and form cations, while atoms with high electronegativities are more likely to accept electrons and form anions.**
Check your Understanding
Try these little questions on your own and see how you do! It's just to see how well you understood this key topic:
Atoms of Ca combine with atoms of Br to form an ionic bond.
- What ratio would they combine in?
- What other compounds have this same ratio with Ca?
- What elements could form an ionic bond with sulfur?
Answers
- Calcium and bromine bond in a 1:2 ratio, creating the compound CaBr2. This is because Ca has a +2 charge and Br has a -1 charge. In order for the two to bond together and form a neutral compound, there must be two Br atoms.
- We need other elements that have a -1 charge in order for them to bond in this 1:2 ratio with calcium. This includes all of the group 17 elements on the periodic table, such as fluorine, chlorine, bromine, and iodine.
- In a 1-to-1 ratio, any elements in group two would form an ionic bond with sulfur. Some examples include MgS, CaS, and BaS. In a 2-to-1 ratio, any elements in group one would form an ionic bond with sulfur. An example is Na2S.
🎥 Watch AP Chemistry teacher Mónica Gracida discuss how to do electron configurations of transition metals and how to quickly know the valence electrons for atoms.

© 2024 Fiveable Inc. All rights reserved.