<< Hide Menu
Dalia Savy
Jeremy Kiggundu
Dalia Savy
Jeremy Kiggundu
A cool thing about the periodic table is that it is organized to demonstrate different trends and properties of elements that can be explained by the pattern of electron configurations and the presence of electron-filled orbitals. The periodicity of the periodic table, or its tendency to recur at intervals, can help you estimate the properties of atoms that haven't even been discovered yet.
For the sake of the AP Chemistry exam, rather than only understanding the trends, you should be able to explain why they happen.
Foundational Concepts for Periodic Trends
In order to fully understand why the trends occur the way they do, it's important to cover the following topics:
Organization of the Periodic Table
As mentioned before, the periodic trends aren't too difficult to grasp since they follow the chronology of the periodic table. It was purposely made to group chemicals of similar properties together.
It is also important to note that the periodic table is divided into 18 columns (called groups) and 7 rows (called periods).
Periods on the Periodic Table ➡️
🤨Properties that differ: Going horizontally, each period is organized in order of increasing atomic number. The atomic number, or the number of protons in an atom's nucleus, determines the basic chemical properties of said element. This trend contributes to the differing effective nuclear charge of elements in the same group, which we'll discuss below.
🤝Shared Properties: In each row, the elements have the same number of occupied electron shells.
Let's compare sodium, which is the first element in period 3, with argon, the last element in period 3.
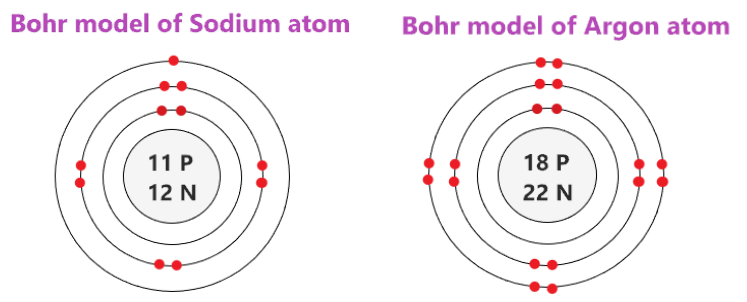
Image Courtesy of topblogtenz
You could see here that both sodium and argon have a total of three occupied electron shells, following the pattern of elements in the same period. However, sodium has 11 protons (represented by its atomic number of 11), and argon has 18 protons (represented by its atomic number of 18).
Groups on the Periodic Table ⬇️
🤨Properties that differ: One group on the periodic table is organized so that as you move down a group, the number of occupied electron shells increase.
🤝Shared Properties: Every element in one group has the same number of valence electrons in its outermost shell. Because these elements all have the same number of valence electrons, they can bond to other elements in similar ways. In other words, these elements tend to have similar chemical properties.
Some groups on the periodic table have a name, since the elements in a single group have similar properties. For example, the elements in group 18 are called noble gases. All noble gases are generally unreactive due to their high stability.
Let's now compare neon, the second noble gas in group 18, with xenon, the fifth noble gas.
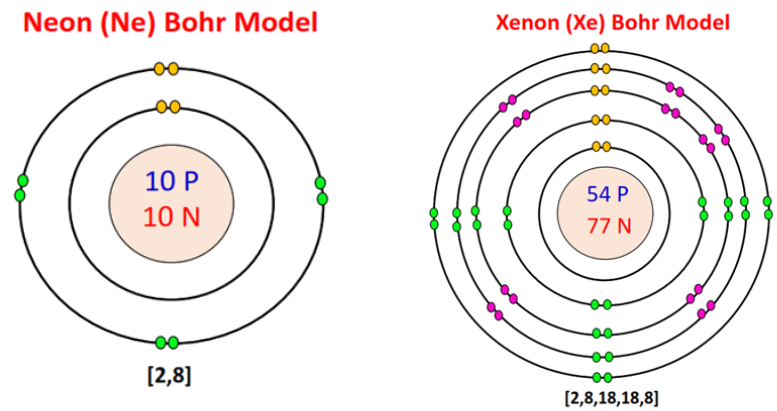
Image Courtesy of topblogtenz
Both neon and xenon have eight valence electrons in their outermost shell, but neon only has two occupied electron shells, while xenon has five occupied electron shells. The fact that they both have a full octet, or eight valence electrons in their outermost shell, makes them both noble gases. Having a full octet makes these elements very stable, and therefore unreactive.
Effective Nuclear Charge
What two subatomic particles make up the nucleus? Protons and neutrons, right? Since neutrons are neutral, protons are the particles that contribute to the positive charge of the nucleus, or the actual nuclear charge (Z).
Now, try to connect this to Coulomb's law which calculates the attraction between two atoms. Each electron orbiting the nucleus experiences both an attraction to the nucleus and a repulsion from the atom's other electrons.
** In order to fully comprehend the forces electrons feel, remember that opposite charges attract. Electrons are negatively charged, and the nucleus is positively charged.**
Electrons that are in the outer shells of an atom may be shielded by the innermost electrons because of the electron-electron repulsion present. In order to accurately represent the nuclear charge of a nucleus, we must account for both the actual nuclear charge and the charge shielded by other electrons (S).
You do not need to know this formula for the AP exam, but it may help you better understand nuclear charge. Effective nuclear charge is equal to the actual nuclear charge (Z) - the charge shielded by other electrons (S).
5 Periodic Trends to Know for AP Chemistry
Let's try to apply the concepts above to the five periodic trends that you should learn and understand for the AP Chemistry exam. The best way to conceptualize this information is to think about it through the concepts we went over above. When in doubt, think about nuclear charge and the periodicity of the periodic table.
Atomic Radius
The atomic radius of an atom is the distance between an atom's nucleus and its valence electrons.
Across a Period - Smaller
Going from left to right on the periodic table, the atomic radii get smaller. As you go right, the atomic numbers increase. This means that there is a higher nuclear charge which increases the pull the nucleus has on the electrons. The closer the electrons are to the nucleus, the smaller the distance.
This trend can also be explained by the fact that all elements in a period have the same number of shells. For example, both Li and F have 2 shells, like Na and Ar both have 3 shells.
Down a Group - Larger
As you go down a group on the periodic table, the atomic radii increase. This is because the number of occupied shells increases. For example in group 1, Li has 2 occupied shells while Cs has 6 occupied electron shells (similar to the trend explained above with neon and xenon).
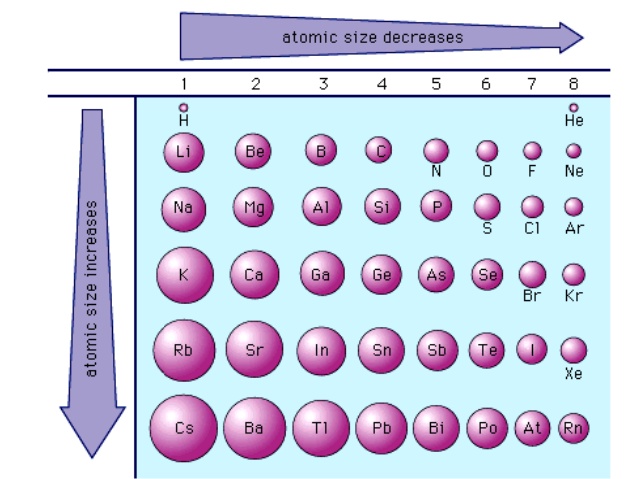
Image Courtesy of Thivyaapriya
Ionic Radius
The ionic radius is the distance between the nucleus of an ion and the valence electrons of that said ion.
➕ Ions < Atoms
- When metals ionize, they lose an electron and become positive ions. Losing an electron makes the ion decrease in size. There is also less shielding and electron-electron repulsion present, allowing the remaining valence electrons to be closer to the nucleus. - Sometimes, metals lose their entire valence shell, significantly decreasing their size.
➖ Ions > Atoms
- When nonmetals ionize, they gain an electron and become negative ions. Gaining an electron makes the ion increase in size. There is also more electron-electron repulsion present due to the increased number of negatively charged particles.
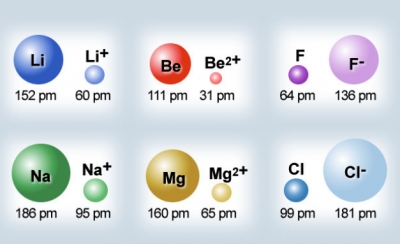
Image Courtesy of Dublin City Schools
Electronegativity
Electronegativity refers to how strongly a nucleus attracts electrons of another atom.
Across a Period - Increases
This is because the elements on the right side of the periodic table (such as noble gases in group 18) have more protons in their nuclei, which gives them a greater positive charge. Having a greater nuclear charge makes the nuclei more effective at attracting electrons.
Down a Group - Decreases
As you go down a group, the atomic size of an atom increases. Therefore, the nucleus of one atom is farther away from the electrons of another atom, and the attraction between the two is weaker.
** Tip - Fluorine is the most electronegative element on the periodic table, with a value of 4.0. Just remember that and try to compare other elements to where fluorine is located on the periodic table.**
Ionization Energy
Ionization energy is the amount of energy needed to remove the valence electrons of an atom. Since there are often multiple valence electrons, there are multiple ionization energies. The first I.E. is the amount required to remove the most loosely held electron and the second I.E. is the amount required to remove the second most loosely held electron.
Across a Period - Increases
Since size decreases across a period, the nucleus and the electrons are more closely attracted to each other. This stronger attraction makes it harder to remove a valence electron. Thus, it takes more energy to do so.
Down a Group - Decreases
As you go down a group, the amount of occupied electron shells increases. The valence electrons that are farther away are more loosely attracted to the nucleus. Therefore, it takes less energy to remove them.
Information to Note
- The 2nd I.E. will always be greater than the first since inner electrons are more strongly attracted to the nucleus.
- There are some exceptions to this trend!- 1st I.E. for group 15 > 1st I.E. for group 16.###### Image Courtesy of Quora- This highlighted electron is in an already occupied orbital. The shielding experienced will lower the required energy to remove the outermost electron, making the 1st I.E. lower than expected for S.- I.E. of Be>B and Mg>Al because of something called quantum tunneling.- Quantum Tunneling - The 2p electron in B is easier to remove than a 2s electron in Be. This is because the 2p electron spends more time away from the nucleus while the 2s electron may tunnel towards the nucleus for enough time to make it more difficult to remove.
Valence Electrons
- Using the ionization energies given below, determine the number of valence electrons this element has.1. I1 = 5001. I2 = 15001. I3 = 70001. I4 = 9000 When you are given this type of question, just look for the huge jump in ionization energies. This gap occurs because of how much more energy it takes to remove electrons closer to the nucleus.
Therefore, this element has 2 valence electrons!
Electron Affinity
Electron affinity is the energy change when an electron is added to an atom in the gaseous state.
Across a Period - more negative
Down a Group - more positive
The more negative the energy, the more energy is released! Electron affinity is typically negative just because an atom releases energy when it gains an electron. However, how negative depends on this trend. You may be able to explain this trend by thinking about electronegativity.
Because of this, you may expect flourine to have the highest magnitude of electron affinity. However, chlorine does! Flourine is too small of an atom and the electrons are so close together that they would repel, which takes energy.
Overview Image of Periodic Trends
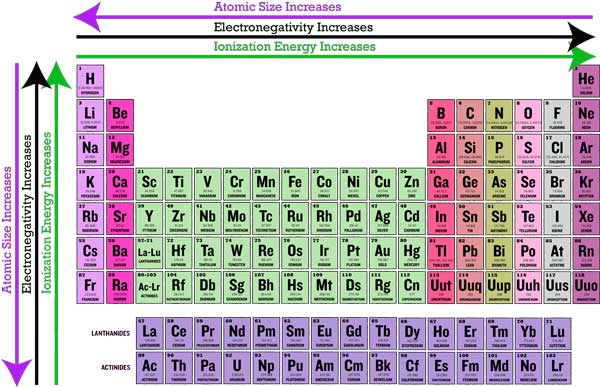
Image Courtesy of Sciencetute
👉 Watch Jacob Jeffries discuss and demonstrate the periodic trends, as well as go over density.

© 2024 Fiveable Inc. All rights reserved.