<< Hide Menu
Dylan Black
Dylan Black
What is Entropy?
In unit 5 you were introduced to the world of thermochemistry. That is, applying the rules of thermodynamics to our study of chemistry! During this unit, you learned about one key measure of energy: heat or enthalpy. In unit 9, our view of thermodynamics will be expanded past simply heat to measure reactions in terms of two other measures: entropy and Gibbs’ Free Energy. In doing so, we’ll explore a reaction’s spontaneity. In simple terms, a spontaneous process will occur without an outside intervention like adding energy to the system. For example, a ball rolling down a hill is a spontaneous process because it occurs without needing anything to happen. However, the reverse process, rolling the ball back up, is non-spontaneous. It takes work to roll a ball up a hill. In this section, we’ll dive into the idea of entropy.
Entropy can be thought of as a measure of “chaos” in a system. Essentially, entropy answers the question of “how ordered is this system?” Entropy is also described as the number of possible arrangements in a system. Essentially, the more spread out and chaotic the system is, the higher the entropy.
Entropy is important to chemists because it helps them understand energy flows in terms of creating order or disorder, especially when discussing reversible processes. To understand this, you can think about a practical example: your bedroom can get messy pretty easily. You can ruin the sheets, punch holes in the wall, throw clothes everywhere, get crazy (Fiveable does not endorse dirty rooms. In fact, go clean your room). This process is a process in which entropy increases. The room went from ordered to disordered meaning entropy increased. You can also use your energy to clean your room. This would mean your room would go from disordered to ordered and the entropy of your room would decrease. This situation explains how systems tend towards chaos—it didn’t take energy to ruin your room but it did take energy to clean it. This is part of the Second Law of Thermodynamics which we’ll discuss in more detail later in this guide.
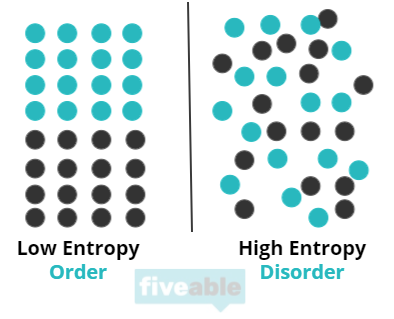
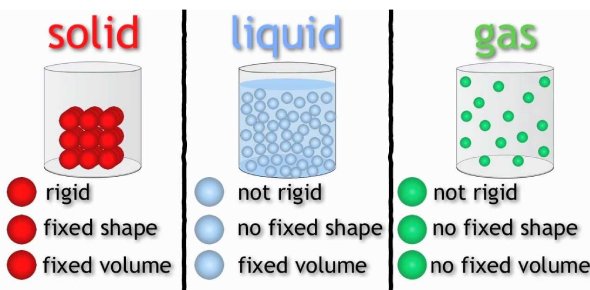
Entropy and States of Matter
There are meaningful connections between entropy and states of matter as well. In general, solids have the least amount of disorder, followed by liquids, and finally, gases have the most entropy. This makes sense when you think about the properties of solids, liquids, and gasses. Solids are tightly packed in and have little movement between the pieces of the solid (either atoms or molecules). Therefore there is the least amount of entropy. Moving to liquids, we see more movement in the molecules and more disorder. Finally, gases are extremely chaotic. Remember that in a gas the molecules theoretically are wildly flying around. Compare this to a solid where there is not much movement at all.
We can represent state changes in the following way:
X (s) ⇌ X (l) ⇌ X (g)
Moving forward along this chain increases entropy and moving backward decreases entropy.
The Three Laws of Thermodynamics
For unit 9, there are three fundamental laws of thermodynamics that you will want to know. You will not have to memorize them verbatim for the AP exam but can be applied to explanations for problems and will give a greater conceptual understanding of the material that will be invaluable.
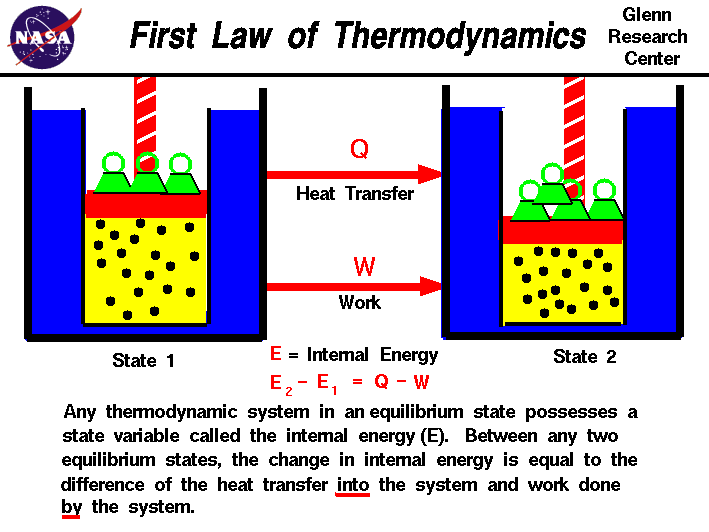
Law #1: The Law of Conservation of Energy
You’re probably already familiar with the First Law of Thermodynamics. This law is also known by a more common name: the Law of Conservation of Energy. This tells us that energy can never be created or destroyed but rather can only change form. For example, energy can change from potential energy to kinetic energy, but the energy between the system and the surroundings is constant. Anything lost to the system is gained by the surroundings and vice versa. From this we also know that the total energy of an isolated system remains constant.
Law #2: Energy Quality and Entropy Increases In Isolated Systems
As far as our discussion of entropy goes, the second law of thermodynamics is perhaps the most important. There are two main parts of this law.
The first part of the second law of thermodynamics refers to energy quality. It tells us that as energy is converted from form to form and/or transfers from body to body, some of it is lost to the surroundings as heat. For example, suppose a power plant runs a turbine to create electricity. In that case, some of the mechanical energy of the turbine is converted to electrical energy while some of it is lost as heat energy via friction. This same idea is applicable to any energy usage.
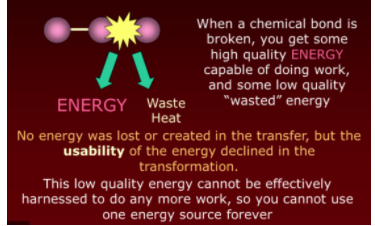
The second part of the second law of thermodynamics refers to entropy changes in an isolated system. The second law of thermodynamics tells us that in an isolated system, entropy will tend to either increase or stay constant. The second law of thermodynamics states that for any spontaneous process, the overall ΔS must be greater than or equal to zero.
Law #3: Absolute Zero
The third law of thermodynamics is the least important for unit 9 but is still incredibly important. This law tells us that at absolute zero (0K = -273.15°C), entropy is a constant zero. This is because at absolute zero, all molecular motion stops and there is no disorder. The formal definition of this law is that “the entropy of a system approaches a constant value as its temperature approaches absolute zero.”

© 2024 Fiveable Inc. All rights reserved.