<< Hide Menu
Dylan Black
Jillian Holbrook
Dylan Black
Jillian Holbrook
In this section, we review what buffers are and how they form, including why we care about buffers in the first place. For chemistry, we often look for solutions with unique properties, whether that be a specific compound, reaction, or observation made. In the case of buffers, these solutions resist changes in pH. This means that adding strong acids or strong bases to them does not impact the pH as much.
It is important to note, however, that buffers are not immune to changes in pH and do have a certain buffer capacity that we will talk about later.
Get it? Buffering? We're hilarious. Image from GIPHY
Buffers Review
As we mentioned, buffers are special solutions that are resistant to pH changes when adding acids or bases to them. Buffers are formed in a very specific way: creating a solution of a weak acid and its conjugate base (or a weak base and its conjugate acid, but the former is much more common).
It is important that the acid you create a buffer with is weak because otherwise, the conjugate base would not be a significant base. For example, a mixture of HCl and NaCl would not be a buffer despite being a combination of an acid (HCl) and its conjugate base (Cl-).
You may be asking then why any weak acid isn't a buffer. At equilibrium, there is so much more acid than the conjugate base (assuming a low Ka) that the buffer effects are negligible. In order for a buffer to be effective, you must have comparable concentrations of acid and conjugate base. In fact, the maximum buffer, the point at which the buffer most effectively resists pH change, occurs when the concentration of acid is equal to the concentration of the conjugate base.
Solidify this concept by doing a few practice problems. For each of the pairs of compounds given, identify them as a pair that would form a buffer or not form a buffer:
-
NaOH and Na+: - The answer to this question is no. Although NaOH and Na+ are a base-conjugate acid pair, remember that NaOH is a strong base. This means that Na+ is not a significant acid and will not form a buffer.
-
CH3COOH and Ca(CH₃COO)₂: - The answer to this question is yes! When dissolved together, this pair will form a buffer. CH3COOH is a weak acid (acetic acid AKA vinegar) with a Ka=1.8 * 10^(-5). Ca(CH3COO)2 is calcium acetate, which will dissociate into Ca2+ (a spectator ion as far as the buffer is concerned), and two moles of CH3COO-, the conjugate base of CH3COOH! Because acetic acid is a weak acid, CH3COO- is a significant base, meaning that we will have a buffer.
-
NH3 and NH4NO3: - This pair does form a buffer. NH3 is a weak base, and NH4+ is a significant acid (and its conjugate acid), meaning this pair forms a buffer. In this case, like Ca2+ in the previous example, the nitrate ion is simply a spectator.
-
HI and I: - Like example one, this pair does not form a buffer. HI is a strong acid and cannot form buffers with its conjugate base I- because I- is not a significant base.
-
KI and PbNO3: - It should be pretty easy to see that this pair does not form a buffer. There are no acids or bases involved. In fact, when you mix KI and PbNO3, you get the "golden rain" reaction, a precipitation reaction that forms PbI2 and KNO3. Take a look!

What Makes Buffers Cool: pH Resistance
Why do buffers have pH resistance, and what makes them so interesting and useful to study? Buffers have pH resistance because of the presence of an acid and a base that do not actively react together at equilibrium. This graphic shows what happens when an acid or a base is added to a buffer:
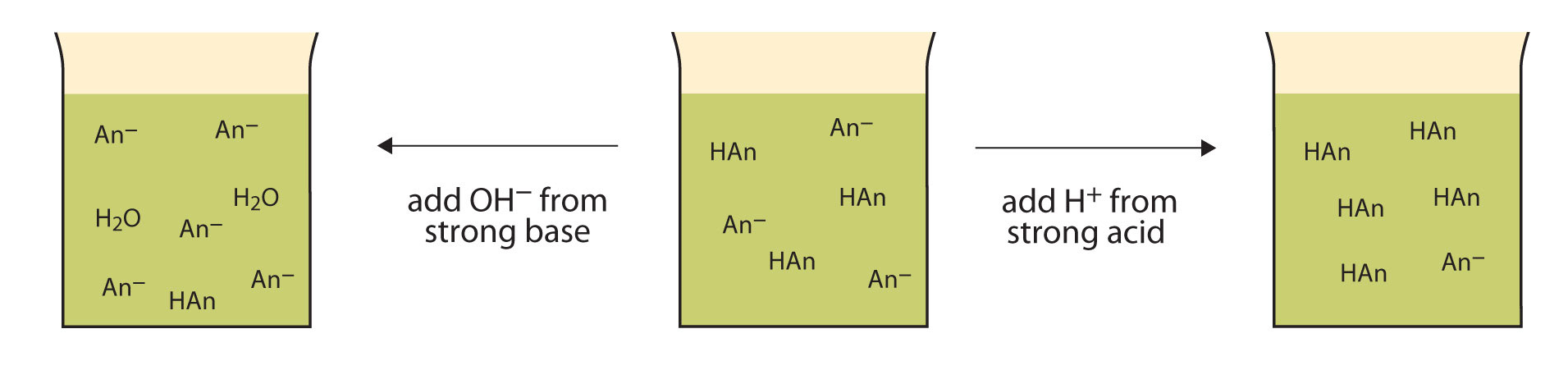
When a strong acid is added to a buffer, the conjugate base eats it up and forms HAn (An = anion). In the case of no buffer, the strong acid would completely dissociate into H+, increasing [H+] to a much higher degree. Similarly, if OH- from a strong base is added to a buffer, the HAn present in the solution reacts with it to form An- and H2O instead of letting it produce pure OH-. These two reactions lead to buffers being resistant to pH!

© 2024 Fiveable Inc. All rights reserved.