<< Hide Menu
Dylan Black
Jillian Holbrook
Dylan Black
Jillian Holbrook
There are many measures in AP Chemistry, especially relating to acids and bases and equilibrium. In this section, we will discuss the relationships between pH and pKa, two of the most important measures!
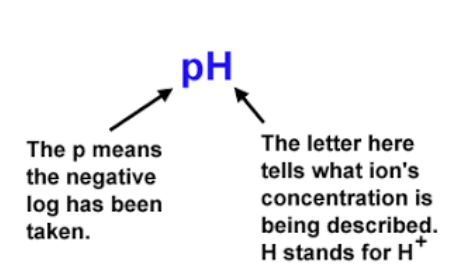
‘p’ Notation
'p' notation is actually fairly simple and seen throughout acid-base chemistry (we have pH, pOH, pKa, pKb, and many more!). 'p'-something is simply equal to the -log(something). For example, pH = -log(H+), and pOH = -log(OH-). Similarly, pKa = -log(Ka).
pKa and Acid Strength
An important use of pKa is in describing acid strength relative to other acids. For example, if one acid has a pKa of 3 and another has a pKa of 2, we know that the acid with a pKa of 2 is 10 times as acidic (note, however, that this does not mean that the pH is 10 times lower). Using 'p' notation gives us a logarithmic scale and not a linear one.
Like pH, where a lower pH corresponds to a higher [H+], a lower pKa implies a higher Ka. However, it is worth noting that a high pKa does not imply basicity. Another note is that, like pH and pOH, pKa + pKb = 14.
pH, pKa, and Buffers
pH and pKa are also related to buffers. As a reminder, a buffer is a mixture of an acid and its conjugate base and is important because it is resistant to changes in pH. However, a question arises: when is the buffer the strongest?
The Henderson-Hasselbalch Equation can be applied to find the pH of a buffer:

The strongest buffer occurs when the concentration of [A-] is equal to [HA]. In this case, pH = pKa + log(1) ⇒ pH = pKa. The relationship is vital, especially when looking at titration curves, because this same point occurs at the half-equivalence point, implying that you have the optimal buffer at the half-equivalence point.
Acid-Base Indicators
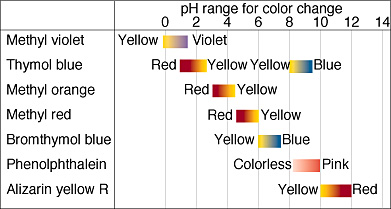
Finally, we will discuss acid-base indicators. Acid-base indicators are a class of compounds that change color depending on the pH of the solution they are in. You may have used indicators in class during titrations to note when the equivalence point of a titration occurs. Some examples of acid-base indicators are bromothymol blue, phenolphthalein, and methyl red.
When choosing an acid-base indicator, you usually want to pick one in which your pH will end up in the effective range, which is the pKa plus or minus 1. While you will not need to memorize any indicators or their effective ranges on the exam, you may be asked to pick which one is the most effective for a certain experiment.
Let's see this concept with an FRQ from 2010:
We are given the following prompt:

In order for the indicator to be useful to us, we want it to change color at the equivalence point for this titration. Looking at the graph, we see that the pH at the equivalence point is 7. We also know this because it is a strong acid strong base titration. Therefore, we want to pick an indicator with a pH range closest to 7. This turns out to be methyl red, which is the correct answer.

© 2024 Fiveable Inc. All rights reserved.