<< Hide Menu
Jillian Holbrook
Jillian Holbrook
Types of Acids and Bases
Arrhenius Definition
There are two main schools of thought for what should be the definitions of acids and bases. The Arrhenius definition categorizes an acid as any compound that increases the concentration of hydrogen ions ([H+]) in a solution. Conversely, the Arrhenius definition of a base is any compound that increases the concentration of hydroxide ions ([OH-]) in solution. Essentially, the Arrhenius definition of an acid/base is anything that yields H+ or OH- respectively in water.
For example, HCl (hydrochloric acid) can be described as an Arrhenius acid because, in water, the following reaction occurs:
HCl → H+ + Cl-
Thus, HCl yields an H+ ion in water, making it an Arrhenius acid.
The concentrations of hydronium ions and hydroxide ions are often reported as pH and pOH, respectively:
pH = −log[H3O+]
pOH = −log[OH−]
pH of Water
Water autoionizes, meaning a proton is transferred from one water molecule to another to produce a hydronium ion (H₃O⁺) and a hydroxide ion (OH⁻), with the equilibrium constant Kw:
Kw = [H3O+][OH−] = 1.0 × 10−14 at 25°C In pure water, pH = pOH, making it a neutral solution because pH = pOH = 7.0. However, the value of Kw is temperature dependent, so the pH of pure, neutral water deviates from 7.0 at temperatures other than 25ºC.
Brønsted-Lowry Definition
Meanwhile, the Brønsted-Lowry definition of acids and bases defines acids and bases in the form of a donation reaction. Basically, an acid/base is seen as an H+ donator or accepter (the acid donates, the base accepts):
HA + B- → HB + A-
In this example, HA is the acid, which donates an H+ ion to the B- ion (the base) to form HB and A-.
Get used to seeing HA and B- as sample acids and bases; it is a common notation! A and B could be stand-ins for any number of ions.
Quick thinkers may be wondering about the reverse reaction in the case that HA + B- is an equilibrium reaction:
HA + B- ⇄ HB + A-

The Hydronium Ion
A consequence of Bronsted Acids and Bases is that when an acid dissolves in water, it needs something to donate its H+ to! In this case, it donates it to water, creating H3O+ or hydronium. Thus, we can see the dissolution of an acid both in an Arrhenius sense as HA <--> H+ + A- and a Bronsted sense as HA + H2O <--> H3O+ + A-. Both of these essentially mean the same thing (and when we learn pH, we'll find that [H+] = [H3O+]), but they display the difference between Arrhenius and Bronsted Acids.
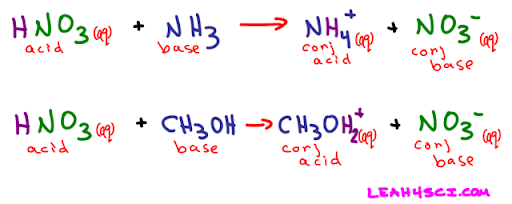
Conjugate Acids and Bases
How do we classify HB and A- then? Would A- be a base and HB be an acid if the reaction were reversed? The answer is short: yes. However, they are given a special name: HB is called the conjugate acid of B-, and A- is called the conjugate base of HA.
In the case that HA is a weak acid and B- is a weak base, HB and A- would be considered to have significant acidity/basicity. The conjugate acid/base of a strong base/acid does not have acidity/basicity. The weaker the acid/base, the stronger the conjugate base/acid.

© 2024 Fiveable Inc. All rights reserved.