<< Hide Menu
8.6 Molecular Structures of Acids and Bases
2 min read•june 18, 2024
Dylan Black
Jillian Holbrook
Dylan Black
Jillian Holbrook
Describing Acid and Base Strength
The following big idea is all about looking at the molecular structure (think Lewis diagrams) of acids and bases to determine their strength. Previously, we learned that there are strong acids/bases and weak acids/bases. How can we differentiate these visually? The first step in using molecular structures to draw conclusions about strength is understanding how we can describe acids and bases as strong/weak based on the molecules themselves.
Essentially, strength stems from the stability of the conjugate base/acid of the acid/base in question. Remember that when describing the strength of a conjugate acid/base, it is inversely proportional to the strength of the base/acid itself. That is to say that the stronger the acid, the weaker the conjugate base. If our conjugate base is a very strong base, our acid is not very strong, and vice versa. To know if a conjugate base is very strong, we discuss the stability of the compound. The question we want to answer for conjugate bases is 'will this compound attract an H+ ion?" For conjugate acids, 'will this compound readily donate an H+ ion?'
Connecting Strength to Structure
Because we now understand how the strength of a conjugate base/acid relates to the strength of its parent compound, we are ready to move into looking at different structures.
One of the first ideas is that weaker bonds to an acidic H will lead to a stronger acid because that bond is more easily broken. For example, in halogenic hydrides (eg. HF, HI, HBr, HCl), the weakest H-X interactions occur with larger halogens. Therefore, moving down the halogen, the acids become stronger because larger atoms will give weaker interactions.
Similarly, acid strength increases from left to right across a period and increases going down a group. This can actually help us understand why strong acids have very weak conjugate bases. When a strong acid, like HCl, dissociates, the conjugate base, Cl-, is incredibly stable. Cl- is a stable ion that will not readily react with much. Ultimately, it is not a reactive base and will create a more dissociative acid.

When discussing oxyacids, the structure can be described by looking at the polarity of the bond between the acidic oxygen (the oxygen attached to the acidic hydrogen). The easier it is for that O-H bond to break, the stronger the acid. In the following image, we represent the "rest" of the acid as "Z."

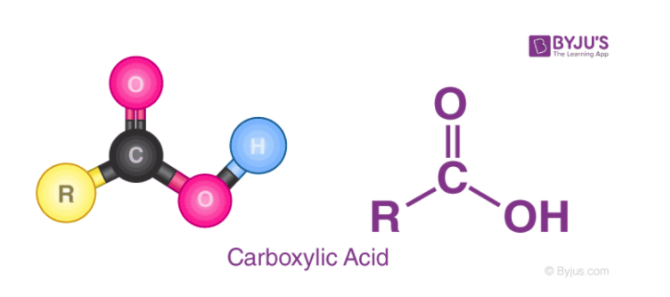
The OH bond is easy to break when Z is either very electronegative or has a high oxidation state. An example of a class of weak acids that are oxyacids is carboxylic acids. Carboxylic acids have a COOH group on the end, such as CH3COOH. Because of the low electronegativity on the carbon, making the bond less polar, these acids are relatively weak.

© 2024 Fiveable Inc. All rights reserved.