<< Hide Menu
Dylan Black
Jillian Holbrook
Dylan Black
Jillian Holbrook
Ideas of pH and solubility connect directly to the common ion effect and Le Chatelier’s Principle. Remember that pH describes the concentration of hydrogen ions (H+) and, conversely, hydroxide ions (OH-) in a solution. By observing connections between solubility equilibria, Le Chatelier’s Principle, and ion concentrations, we will be able to achieve some insights as to how pH can impact solubility equilibria!
Effects of Acidic Solutions
Like most other conditions, pH has an important effect on solubility, specifically when dealing with compounds that decompose into the conjugate base of a weak acid. Remember that the conjugate base is the ion formed from the dissociation of an acid into H+ and A-, where A- is the conjugate base.
A necessary factor to consider when discussing different conjugate bases is that the conjugate base of a weak acid, one that does not fully dissociate, is basic. The same cannot be said for the conjugate bases of strong acids such as Cl-, Br-, and ClO4-. Why is this important? It means that the conjugate acid of a weak base A- can react with water in the following fashion:
A- + H2O ⇌ HA + OH-
If the dissolution took place in a strongly acidic solution, the H+ would react with A- to form HA. This will decrease [A-], decreasing Q and pushing the dissolution to the right. Therefore, an acidic solution will increase the solubility of a compound that forms the conjugate base of a weak acid. Generally, the more basic the anion (ie. the weaker the acid it derives from), the more soluble a compound will be in an acidic solution. Similarly, compounds that dissolve into hydroxides, such as strong bases, are more soluble in acidic solutions.
However, an acidic compound will be less soluble in an acidic solution due to the common-ion effect. Because of the already present H+ concentration, a soluble acid will be less soluble in an acidic solution.
As an example problem, consider the following reaction:
Fe(OH)3 ⇌ Fe3+ + 3OH-
Will the solubility increase or decrease in an acidic solution? Explain.
In an acidic solution, we will have a high concentration of H+ ions (also seen as H3O+ or hydronium). These H+ ions will react with the OH- ions to form H2O in the autoionization of water. Therefore, in an acidic solution, there will be a lower concentration of OH- ions. According to Le Chatelier’s Principle, decreasing the concentration of OH- will push equilibrium to the right because Q will become less than K. Therefore, in an acidic solution, Fe(OH)3 is more soluble than it is in a neutral solution.
Effects of Basic Solutions
The effects of a basic solution are essentially the opposite of those in an acidic solution. In a basic solution, compounds that dissolve into significant conjugate acids, such as NH4+, will be more soluble in a basic solution. This is because the following reaction will take place:
NH4+ ⇌ NH3 + H+
In a basic solution, the OH- will react with the NH4+ and decrease [NH4+], therefore decreasing Q and pushing the overall dissolution towards the products.
On the other hand, basic compounds such as strong bases like NaOH or compounds that dissolve into significant conjugate bases will be less soluble. For strong bases, such as Ba(OH)2, this is because of the common ion effect. The already present OH- concentration will serve as a common ion in the solution.
For weak conjugate bases, such as the case of CH3COO-, the reaction of CH3COO- and H2O forming OH- and CH3COOH will be shifted left because of Le Chatelier’s Principle. Thus, in a basic solution, there will be less solubility for compounds with conjugate bases as ionic components.
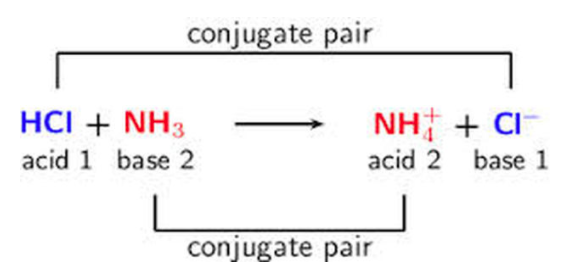
Image From Acids and Bases 101
pH Neutral Compounds
An important note is that compounds that do not have any basicity or acidity are not impacted by pH. For example, NaCl dissociates into Na+ and Cl-, the conjugate acid of NaOH and the conjugate base of HCl, respectively. However, note that Na+ and Cl- are both insignificant. This is because they are the conjugates of strong acids/bases. Therefore, they will not be impacted by any acidity or basicity of the solution. The only time solubility may be affected by the presence of an acid or base is in the presence of a common ion, but this is not connected to pH itself.
Learning Summary ✏️
Although computations of solubility as functions of pH will not be tested on the AP Exam, the test expects a solid understanding of the qualitative effects that changes in pH have on the solubility of salts. In this topic, we learned how acidic conditions, basic conditions, and neutral conditions affect solubility in the context of Le Chatelier’s principle!

© 2024 Fiveable Inc. All rights reserved.