<< Hide Menu
Dylan Black
Dalia Savy
Dylan Black
Dalia Savy
Reversible Reactions
Welcome to the introduction to equilibrium! Equilibrium is an incredibly important topic both for the AP exam and for chemistry in general. Equilibrium takes what you know about reactions from unit 4 and unit 5 and expands it into the realm of reversible reactions. That is reactions that can go both forward (reactants turning into products) and backward (products turning into reactants) at the same time. This unit will focus on describing how reactions can do this and to what extent they do.
Many observable processes in nature are reversible, such as:
- The evaporation and condensation of water; H₂O(l) ⇌ H₂O(g)
- The dissolution and precipitation of a salt; NH₄Cl(s) ⇌ NH₄⁺(aq) + Cl⁻(aq)
- Acid-base reactions; H₂CO₃ + HCO₃⁻ ⇌ H₂O + CO₂
- Redox reactions; Zn + Cu²⁺ ⇌ Zn²⁺ + Cu All of these reactions can go back and forth, forming both products and reactants.
Arrows in Chemistry
The concept of reversible reactions introduces a new arrow that you can use: the double arrow (⇌). This symbol is used in a chemical equation of a reversible reaction and also indicates a system in equilibrium, which we will discuss soon.
When you are completing the free-response questions on the AP Chemistry exam, make sure you are using the right arrow:
- The single arrow shows a reaction proceeding in one direction (→)
- The double arrow shows a reversible reaction at equilibrium (⇌)
What Is Equilibrium?
Let’s begin by asking the question, “what even is equilibrium?” In most textbooks, equilibrium is defined as the point in a reaction where the rate of the forward reaction is equal to the rate of the reverse reaction. Let’s break this down a bit.
Suppose we have a reaction in which A, a reactant, is turned into B, a product. As concentrations of A decrease, the rate of reaction A → B will decrease. However, there will be a secondary reaction occurring simultaneously, that being the reaction B → A as concentrations of B build up. In many instances, this second reaction is incredibly slow and does not happen much. However, in many other instances, this second reaction is actually the driving reaction. We’ll talk a bit more about how to measure how “far forward” a reaction goes by using equilibrium constants.
Going back to arrows, we can represent the two reactions by using a double arrow on the reaction A → B to describe that this reaction is reversible: A ⇌ B. This notation tells us that this reaction will settle at an equilibrium. Now that we’ve covered that, we’re ready to dive more into how the rates of these two reactions relate.
Representing Equilibrium Graphically
As we mentioned before, equilibrium is the point at which the forward reaction and reverse reactions continue at the same rate. At this point, the production of products equals the production of reactants and so concentrations of the two remain the same. The below graphic shows us what happens at equilibrium:
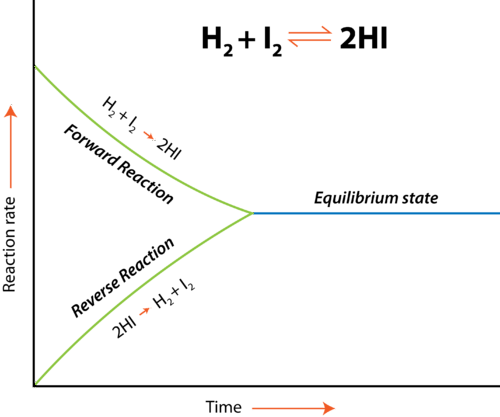
In this example, we have the equilibrium reaction H₂+I₂ ⇌ 2HI. We have two reactions each occurring at different rates, one being the forward reaction and the other being the reverse reaction. However, note the point at which the two become the same. That area is called the equilibrium state. At this point, the rate at which products are created equals the rate at which reactants are created and therefore the concentrations present in your reaction are equal. It’s important to note that equilibrium doesn’t mean the concentrations themselves are the same, but rather just the rates of each reaction.
In sum, at equilibrium, the rate of the forward reaction = the rate of the reverse reaction, and the concentrations of the reactants and products remain constant. Rates are equal, but concentrations are constant.
A Closed System
Equilibrium can only occur in a closed system, which is a system that does not exchange matter or energy with its surroundings. This is because in a closed system, the concentrations of reactants and products are fixed and cannot change. The only way for the concentrations to change is through the forward and reverse reactions. As the forward and reverse reactions proceed, the concentrations of the reactants and products change until they reach a state where the rate of the forward reaction is equal to the rate of the reverse reaction.
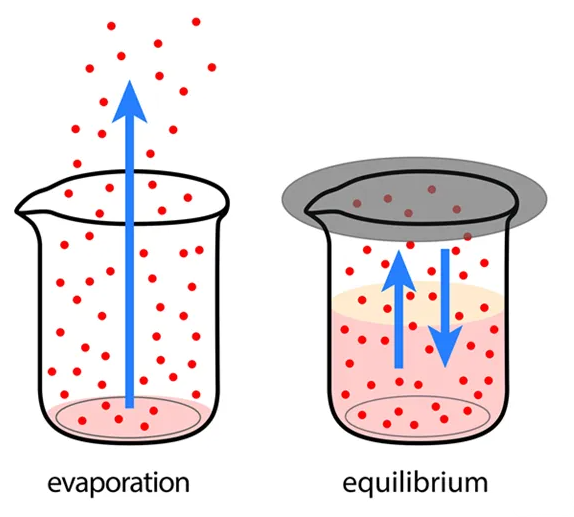
Image Courtesy of Shalom Education
In an open system, matter and/or energy can be exchanged with the surroundings, which can affect the concentrations of the reactants and products, making it impossible for the system to reach equilibrium.
Measuring Equilibrium: Kc
Now that we understand what equilibrium means in terms of reaction rate, let’s discuss how we actually measure how “far forward” a reaction goes. When doing calculations with equilibrium, we use the equilibrium constant, K for a reaction. K describes the ratio between the rate of the forward reaction and the rate of the reverse reaction, as well as the ratio between the equilibrium concentrations of products and reactants. If we have the reversible reaction A ⇌ B, we can describe how far forward this reaction goes before equilibrium by writing out the rate laws for the forward and reverse reactions:
A → B ⇒ R = k₁[A]
B → A ⇒ R = k₂[B]
At equilibrium, k₁[A] = k₂[B]. We can describe the ratio k₁/k₂ as a representation of how far forward the product-creating reaction goes vs. how far forward the reactant-creating reaction goes. We can also represent this value as [B]/[A] where [B] and [A] are the concentrations of B and A at equilibrium. This value is known as Kc or sometimes simply K. The formula for K is as follows:
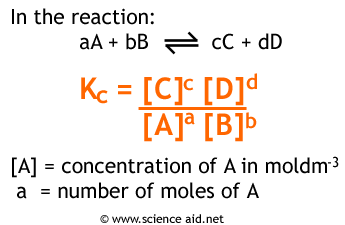
K is a unitless value that changes based on temperature (since k1 and k2 change based on temperature). However, the initial reactant and product concentrations do NOT change K. No matter where you start a reaction (assuming constant temperature), you will find that K remains constant. This will also be true for KP which we’ll discuss next.
Measuring Equilibrium: Kp
There is another way of measuring equilibrium, specifically when discussing gasses. Like we use concentrations, we can also use partial pressures to describe how many products were formed and how many reactants remain at equilibrium. This value is known as Kp. Kp is the same calculation as Kc just with partial pressures instead of concentrations.
In this study guide, we're focusing on what Kc and Kp conceptually represent, but later in this unit, we'll be doing some calculations with them! For now, make sure you’re comfortable understanding what they mean in a non-mathematical way as well like we’ve described here.
There is also a relationship between Kp and Kc that is described by the formula Kp= Kc(RT)^(Δn) where Δn denotes the difference in stoichiometric coefficients of the products and the stoichiometric coefficients of the reactants. This won’t be too common on the exam (in fact it probably won’t show up), but it’s nice to know!
Common Misconception: Equilibrium = Stopped Reaction
Let’s close this guide out with a common misconception that students have about equilibrium that hinders their understanding of equilibrium as a balancing of rates. Many students believe that at equilibrium the reaction stops completely. This could not be further from the truth! In fact, reversible reactions never “end”. Equilibrium is a dynamic process in which reactants are still turned into products and vice versa, not the point at which a reaction stops. What this means is that the reactants and products are constantly interconverting. The system is constantly active, but there is no observable change because concentrations remain constant.

© 2024 Fiveable Inc. All rights reserved.