<< Hide Menu
7.4 Calculating the Equilibrium Constant
5 min read•june 18, 2024
Dylan Black
Dalia Savy
Dylan Black
Dalia Savy
Now that we've got a handle on what Kc and Kp are, we can start using them and calculating for them! If you're still a bit confused about what Kc and Kp are conceptually, check out our study guide introducing them.
When calculating equilibrium constants, you are typically given equilibrium concentrations or are given an easy way to calculate them without using K (however, the former is much more common). At that point, you simply plug into one of the formulas we’ve learned and get your answer!
Remember, K is a unitless quantity. It is important to note however that solid precipitates and liquids are not part of the equilibrium expression. Only aqueous solutions and gases are.
Formulas For Equilibrium Constants
In chemistry, equilibrium refers to the state in which the concentrations of reactants and products in a chemical reaction remain constant over time. The equilibrium constant (Kc) is a value that relates the concentrations of the reactants and products at equilibrium, while the equilibrium constant (Kp) relates the pressures of gases at equilibrium.
Both Kc and Kp have the same formulas, but the way you represent concentrations and partial pressures are different. Make sure you can distinguish between the two, as writing in the incorrect format will not count as full credit on free-response questions.
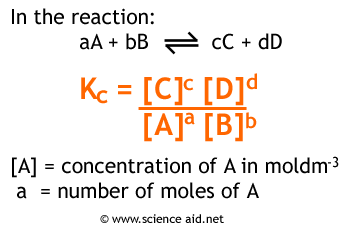
Image Courtesy of ScienceAid

Image Courtesy of Quora
Calculating Kc
Calculate the value of the equilibrium constant, Kc, for the system shown below if 0.1908 moles of CO₂, 0.0908 moles of H₂, 0.0092 moles of CO, and 0.0092 moles of H₂O vapor were present in a 2.00 L reaction vessel at equilibrium.
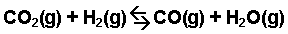
Image Courtesy of Purdue University
First, begin by writing out the Kc expression for this reaction as the ratio of the products over the reactants. Since we are given mole amounts and a volume, we know we can calculate for molarity and use Kc:
Kc = [CO][H₂O]/[CO₂][H₂]
Then find our equilibrium concentrations by dividing each given mole amount by 2.00 L:
CO: 0.0092/2 = 0.0046 M
H₂O: 0.0092/2 = 0.0046 M
CO₂: 0.1908/2 = 0.0954 M
H₂: 0.0908/2 = 0.0454 M
Finally, we can plug into the Kc expression above to calculate Kc:
Kc = [0.0046][0.0046]/[0.0954][0.0454] = 4.9 * 10⁻³.
Note that since each reactant and product had a stoichiometric coefficient of one, we did not have to include any exponents. Also, this reaction was full of gases, so we were able to include each substance in the equilibrium constant.
Calculating Kp
Calculate the Kp for the reaction 2N₂O₅ (g) ⇌ O₂ (g) + 4NO₂ (g), if:
- P(N₂O₅) = 2.00
- P(O₂) = 0.296
- P(NO₂) = 1.70 First, we can write out our Kp expression by recognizing that we were given partial pressures and that all substances in the reaction are gases:
Kp = P(O₂)P(NO₂)⁴ / P(N₂O₅)²
Since there are stoichiometric coefficients other than one, we must account for them in the equilibrium constant. All that is left is to just plug in the values and calculate Kp:
Kp = (0.296)(1.70)⁴ / 2.00² = 0.618
Justifying the Formula For The Equilibrium Constant
Let’s think about why the formula we’ve been using actually works. In a general reversible reaction A + B ⇌ C + D, the equilibrium constant K is equal to the ratio of the equilibrium concentrations of the products raised to their stoichiometric coefficients to the equilibrium concentrations of the reactants raised to their stoichiometric coefficients.
We see this mathematically as K = [C][D] / [A][B]. Let’s think about what this formula actually tells us. Recall that these concentrations are equilibrium concentrations meaning the numbers we plug into this formula are after the reaction reaches equilibrium. By finding a ratio, we’re essentially asking the question, “How does the number of products at equilibrium compare to the number of reactants at equilibrium?”.
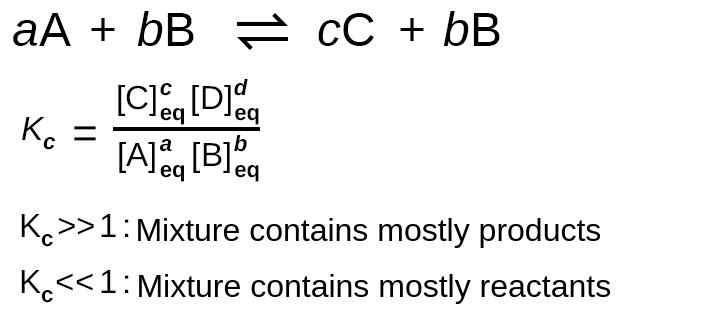
Image Courtesy of Labster Theory
This helps us explain why a K value above 1 indicates a product-favored reaction and vice versa. The formula tells us that when K is over 1, [C][D] > [A][B] meaning that we now have more product than reactant. Similarly, when K is less than 1, [C][D] < [A][B], and thus we still have a large amount of reactant. Note that K can never be negative but can be extremely small. This way of thinking can help you understand why the equilibrium constant formula is the way it is!
Tips When Calculating Equilibrium Constants
While calculating equilibrium constants is usually a plug-and-play game, there are a few things you want to be careful of before blindly plugging into the formula. The most important aspect of the formula is that concentrations and pressures are so AT EQUILIBRIUM! Plugging in pressures at any other point besides at equilibrium will calculate Q, the reaction quotient, which for all but ONE point is not the equilibrium constant. Thus, you have to be super careful that you are actually plugging in values at equilibrium.
You also want to make sure the numbers you’re plugging in are actually concentrations/pressures. For example, look back to the example for calculating Kc. We glossed over this step because it’s assumed prerequisite knowledge for this unit, but you want to make sure that you are converting to the proper units. Using the example we went through as a sample, we see that we had to divide by 2.00L to find mol/L because we were originally given moles.
A problem could in theory take this a step further and give you grams and expect you to convert grams to moles and then moles to moles per liter. Always be prepared to make unit conversions when you have to especially since dimensional analysis is such a fundamental technique in this course.
This could also take the form of calculating partial pressures. For example, if you were given a total pressure and then moles of each gas, you would have to use PA=XA*Ptotal to find each partial pressure, and then plug them into the Kp expression. These instances may be rare but could theoretically pop up because they are part of chemistry.
AP Question - 2017 #3
The following question is part of number three of the free-response section on the 2017 AP Chemistry exam. All courtesy is to College Board.
N₂(g) + O₂(g) ⇌ 2NO(g)
At high temperatures, N₂(g) and O₂(g) can react to produce nitrogen monoxide, NO(g), as represented by the equation above.
(a) Write the expression for the equilibrium constant, Kp, for the forward reaction.
One point is given for the correct Kp expression:
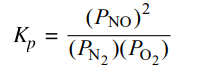

© 2024 Fiveable Inc. All rights reserved.