<< Hide Menu
6.1 Endothermic and Exothermic Processes
7 min read•june 18, 2024
Dalia Savy
Anika P
Dalia Savy
Anika P
Forms of Energy
As you recall from unit four, matter can undergo physical and chemical changes. Chemical changes involve the forming or breaking of ionic and covalent bonds between elements, but physical changes involve intermolecular changes. Such energy changes are related to changes in temperature. This can easily be seen with the melting of ice, which is a physical change:
H₂O (s) → H₂O (l)
🔥 Energy is supplied to melt the ice into liquid water! Energy is the capacity to do work or transfer heat. There are three types of energy that are commonly tested:
Kinetic Energy
Kinetic energy is the energy that results from the motion of an object. We've discussed kinetic energy a lot so far with regard to temperature since the temperature of particles is directly related to the average kinetic energy of the particles in a substance. Therefore, as temperature increases, the gaseous particles move faster. Remember unit three and the behavior of ideal gases?
KE=½mv^2, where mass, m, is expressed in kilograms and velocity, v, is expressed in meters per second.
KE is expressed in Joules. You don't have to memorize this formula as it is on your AP reference sheet, but be aware that mass and velocity impact the kinetic energy of an object.
Potential Energy
Potential energy is the stored energy in an object based on its position. It usually results from both attractive and repulsive forces. In chemistry, we focus on potential energy as the energy stored in the chemical bonds between atoms and molecules.
PE is related to the stability of a compound as well! Compounds with lower potential energy are more stable than those with higher potential energy. This is because, in order for a compound to be stable, the energy of the bonds between its atoms and molecules must be minimized. When a compound is in its most stable state, the energy of its chemical bonds is at its lowest possible value. We've seen this relationship in several graphs so far throughout this course.
Electrostatic Energy
Electrostatic energy is a form of potential energy that results from the interaction of charged particles. You could associate this with Coulomb's Law, which we discussed several times in previous chapters.
PE of an electron = Q1Q2/d, where charges, Q1 and Q2, are separated by a distance, d
Remember, the attraction between ions only occurs if the charges are opposite and repulsion only occurs if the charges are the same. If you have trouble remembering this, think about magnets! 🧲
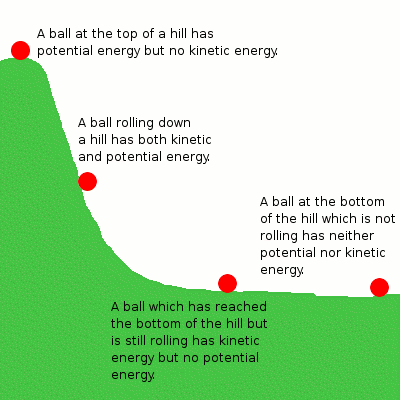
Image Courtesy of NRG
The Law of Conservation of Energy
This unit is based on one central idea: The Law of Conservation of Energy. This fundamental principle in chemistry and physics states that energy cannot be created or destroyed, only transferred or converted from one form to another. This means that the total amount of energy in a closed system remains constant over time. This applies to all forms of energy.
Let's discuss this in terms of the mechanical system above with the example of the ball rolling down the hill. The potential energy the ball has due to its height can be converted into kinetic energy as it falls, but the total energy remains the same. Similarly, in a chemical reaction, the potential energy stored in the reactants is converted into the potential energy of the products, but the total energy before and after the reaction remains the same.
Since it is so so important, it is also called the First Law of Thermodynamics. Since the universe is considered an isolated system, the total amount of energy in the whole universe is constant.
Studying Energy Changes
The System
To analyze energy changes, we first have to be able to define the system. This is a specific part of the universe that is of interest to us and would typically involve substances used in experiments (ex. HCl, NaOH, etc.). There are several types of systems:
- Open System ⚖- An open system always allows air, heat, and energy from its surroundings into it. It is generally an exchange system. For example, if there was an open system at 90 degrees and the air around it was at 50 degrees, heat and energy would exchange until both would reach 70 degrees. This is called a system at equilibrium which you'll learn more about in unit seven. ⚖️1. Analogy: An example of an open system is a pot of water on a stove. The water in the pot can exchange heat energy with the stove and the air around it, and it can also exchange matter, such as steam, with the surrounding air.
- Closed System - A closed system is usually represented as an open system, but with a stopper. This way, air cannot transfer into or out of the system. Rather than mass and heat/energy being able to transfer, only heat is able to transfer.1. Analogy: An example of a closed system is a thermos. The contents of the thermos can exchange heat energy with the surrounding air, but the liquid inside the thermos cannot escape.
- Isolated System - An isolated system is completely covered on all ends to prevent the transfer of mass, heat, and energy. An example of this is a calorimeter, which is key in this unit! Insulations allow for an isolated system as well.1. Analogy: An example of an isolated system is a sealed container filled with gas. The gas inside the container cannot exchange matter or energy with the surrounding air.
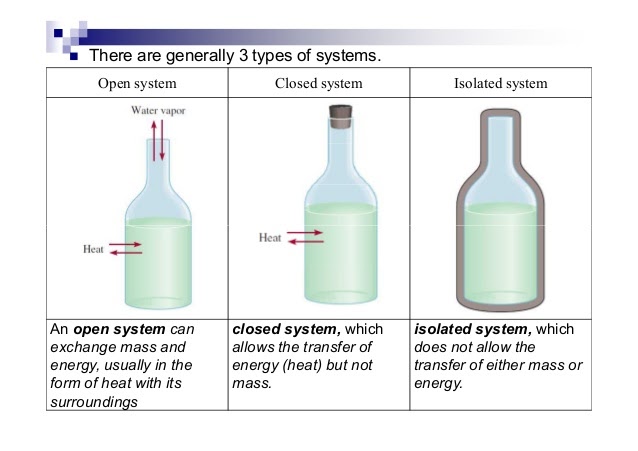
Image Courtesy of Socratic
The Surroundings
The rest of the universe outside the system, such as a beaker, constitutes the surroundings. When taking a look at a question, try to always identify what is in the system and surroundings first.
The system is where the reaction takes place and the surroundings are everything around the system.
State Functions
In thermodynamics, we study changes in the state of a system. State functions, such as energy, enthalpy, pressure, volume, and temperature, are properties determined by the state of the system. It does not matter how the condition was achieved, so focus on the initial and final states of the system only.
Heat and work are not state functions, because they vary on the path to the destination. 🛣️
An analogy to help understand state functions is to think of a road trip. The distance between your starting point and your destination is a state function because it only depends on the starting and ending point and does not depend on the specific route taken or the speed at which you traveled.
Endothermic vs Exothermic
All forms of energy can be described as either exothermic or endothermic processes. We've discussed these terms when looking at potential energy graphs in the kinetics unit, but let's look at them from the perspective of the system and its surroundings.
Before we do that though, we have to define enthalpy. Enthalpy (H) is a state function that describes the total heat content of a system. ΔH (Delta H) is the change in enthalpy of a system or the difference between the final and initial enthalpies of a system. It is a measure of the heat absorbed or released in a chemical reaction or a physical process such as a change of phase or temperature.
🥵 Endothermic processes, as indicated by the prefix endo-, are processes where heat is supplied to the system by the surroundings. This corresponds with a +ΔH value, indicating that heat is absorbed.
-
Remember: endo, positive ΔH, heat travels from surroundings to system. 🥶 Exothermic processes, as indicated by the prefix exo-, are processes that give off heat and transfer thermal energy from the system to the surroundings. This corresponds with a -ΔH value, indicating that the system is releasing or losing heat
-
Remember: exo, negative ΔH, heat travels from system to surroundings.
Think about these concepts from the point of view of the system. If the system is gaining energy, there is a positive change, so it is an endothermic process. If the system is losing energy, there is a negative change, so it is an exothermic process. We'll go over ΔH more later in this unit.
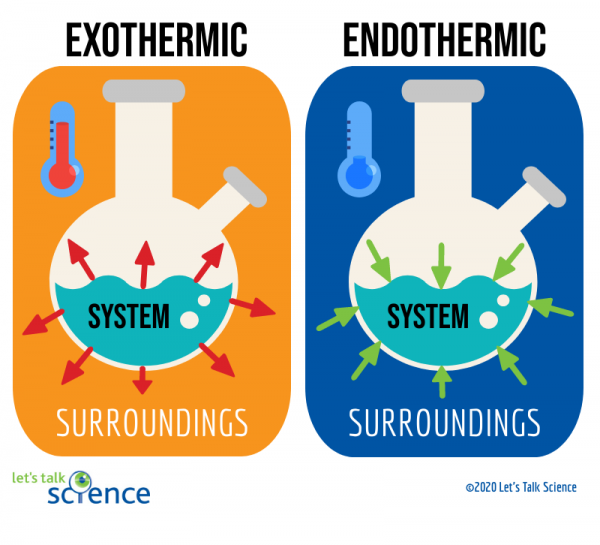
Image Courtesy of Let's Talk Science
Hot and Cold Packs: The Chemistry Behind Them
As you know, hot and cold packs are used to provide therapeutic heat or cold to a specific area of the body. Have you ever wondered how these spontaneous packs work? Well, the chemistry behind these packs involves the use of exothermic and endothermic processes.
Inside these packs, there are two separate bags: one containing water and the other containing a chemical substance. When you crack them, the barrier between the bags break, and the two components react.
An instant hot pack typically contains a substance that can absorb water and undergoes an exothermic reaction when it comes in contact with water. An example of such a substance is sodium acetate, which is a common ingredient in hot packs. When the hot pack is activated by squeezing it, a small amount of water is mixed with the sodium acetate, which triggers an exothermic crystallization reaction, releasing heat into the hot pack and warming you up.
A cold pack, on the other hand, typically contains a substance that can release water and undergoes an endothermic reaction when it comes in contact with water. An example of such a substance is ammonium nitrate, which is commonly used in cold packs. When the cold pack is activated by squeezing it, the ammonium nitrate dissolves in the water, absorbing heat and making the pack feel cold to you.

© 2024 Fiveable Inc. All rights reserved.