<< Hide Menu
Dalia Savy
Anika P
Dalia Savy
Anika P
Bond Energetics
While it may not seem so, bonds contain energy😲. When you think about why some reactions are exothermic and others are endothermic, it is because of the breaking and forming of bonds! There is a general rule here, and that is: BREAKING BONDS REQUIRES ENERGY & MAKING BONDS RELEASES ENERGY.
**I have a mnemonic device you could use to make your life so much easier! BARF: Breaking Absorbing - Releasing Forming. This reiterates that when bonds are broken, energy is absorbed and when bonds form, energy is released. **
Breaking Bonds Requires Energy
Imagine a single molecule of H2. If you wanted to break that bond, you have to input energy. Think of it almost like having to snap a branch in half or stretching a rubber band until it snaps💥. In order to do this, we have to put energy into the system!
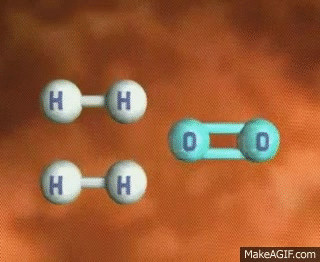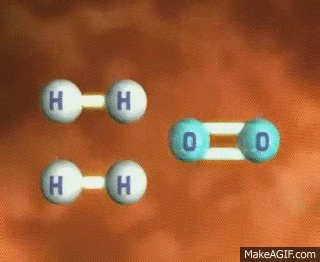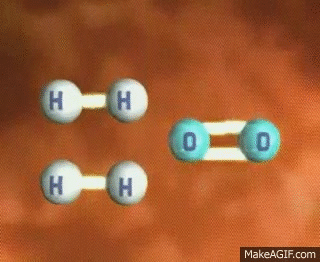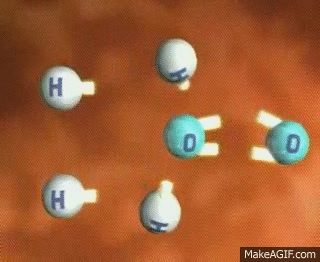
GIF Courtesy of MakeAGIF
Making Bonds Releases Energy
Conversely, when you form a bond, energy is released. This is because of lower potential energy at these states. This leads to excess energy being released from the system. This can be seen in the following chart:
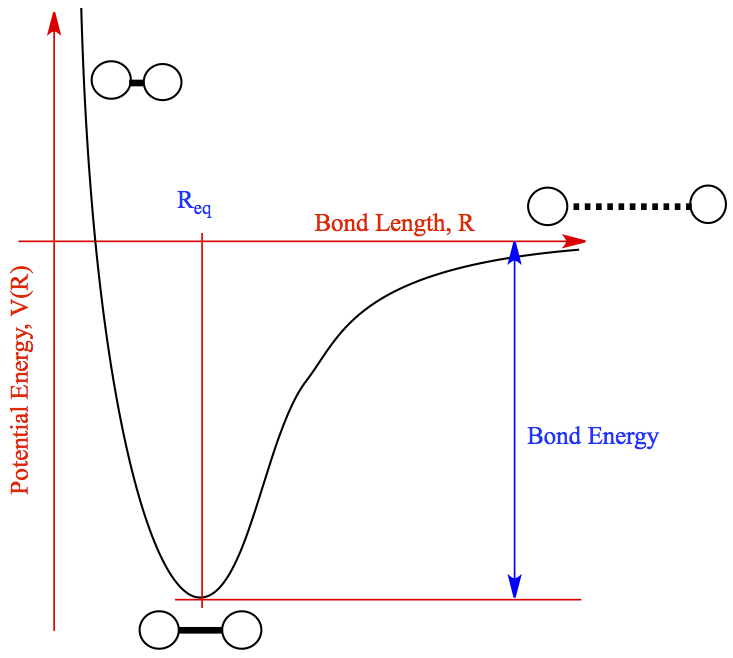
When atoms are bonded together in a correct formation, potential energy is released. When they are too close, repulsive forces make potential energy high and when they are too far apart, potential energy approaches zero since there are no attractive forces.
📝Read: AP Chemistry - Intramolecular Force and Potential Energy
Bond Dissociation Energy
Now that we've figured out that breaking bonds takes energy and that forming bonds releases energy, let's start examining how we quantize this energy. To begin, the energy that it takes to break a specific bond is called the bond dissociation energy, or BDE. This varies from bond to bond, but there are some basic trends:
More Bonds, Higher BDE
Typically, a triple bond has a higher BDE than a double bond than does a single bond. This is because simply put, there's a more robust bond to break through. This should make logical sense, though it is worth noting🧐.
Longer Bond, Weaker Bond
Similarly, a shorter bond will be stronger and a longer bond will be weaker. This is because of potential energy differences based on bond length.
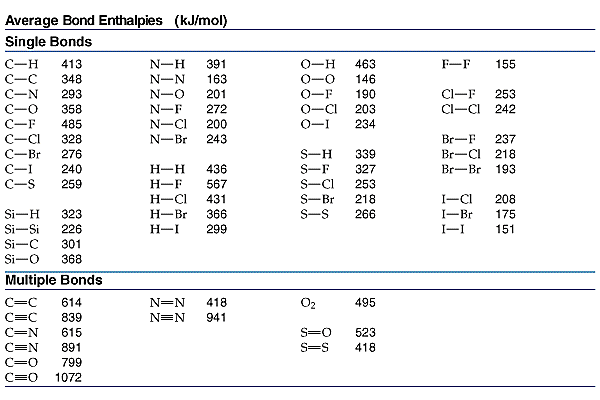
To sum these facts up, shorter bonds are stronger and have a lower BDE compared to longer bonds, which are weaker and have a higher BDE.
Enthalpy of Reaction Using BDEs
Bond Dissociation Energy can be used to calculate the amount of energy released or absorbed during a chemical reaction. This is because of one simple principle: all a reaction is is the breaking of reactant bonds and the forming of product bonds. Therefore, we can use a simple formula:
ΔH = ΣH(broken) - ΣH(formed)
💡KNOW it's broken - formed, or reactants - products. In the next key topic, you are introduced to another formula that is products-reactants, so be careful.
When BDEs are introduced into a problem, automatically think products - reactants.
Breaking Down This Formula
This formula essentially is the sum (that's what Σ, or sigma, means) of the bond dissociation energies of the reactant bonds broken minus the sum of the bond dissociation energies of the product bonds formed. An easy way to do this is to break all the bonds and then, from such, rebuild the products.
Example Problem #1
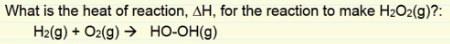
We are given the following thermodynamic data:
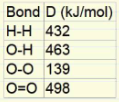
We know that: ΔH = ΣH(broken) - ΣH(formed)
Let's apply this formula: ΔH = (H-H + O=O) - (O-H + O-O + O-H) ΔH = (432 + 498) - (463 + 139 + 463) = -135 kJ/mol
Usually, it is best to draw out the molecules in the reaction because you want to be sure that you use the correct BDE. For example, what BDEs would you use for CO2? You probably said 2(C-O), or two single carbon to oxygen bonds. However, if you draw CO2 out, you would find out that there are actually 2 double carbon to oxygen bonds, which completely changes your answer.
Example Problem #2
Let's try another problem! Find the heat of reaction for the following:
CH4(g) + 2O2 (g) --> CO2(g) + 2H2O(g)
| Bond | D (kJ)/mol |
| C-H | 413 |
| O-H | 463 |
| C-O | 358 |
| C=O | 799 |
| O=O | 498 |
ΔH = [4(C-H) + 2(O=O) ] - [2(C=O) + 4(O-H)]
ΔH = [4(413) + 2(498)] - [2(799) + 4(463)] = -802 kJ/mol
This reaction is an exothermic reaction since the forming the bonds took more energy than breaking the bonds.
Make sure to always put a sign to the ΔH when doing bond dissociation energy questions. If it is an exothermic reaction and you forget the negative sign, points will be deducted😢.

© 2024 Fiveable Inc. All rights reserved.