<< Hide Menu
Jillian Holbrook
Jacob Jeffries
Jillian Holbrook
Jacob Jeffries
🎥 Watch: Catalysis
What is a Catalyst?
Catalysts lower the activation energy of a reaction. Those who are proficient in biology may recognize catalysts as having a similar function as enzymes. Catalysts are defined as species that are consumed in one step in a reaction mechanism but appear again later. Essentially, they go in as a reactant but come out as a product COMPLETELY UNTOUCHED. They do not play a role in the actual reaction but rather modify the mechanism such that the energy regarding the reaction changes.
This is shown through the notation of a catalyst above the reaction arrow, as seen in the following image.
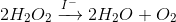
The equation can be read: "The decomposition of hydrogen peroxide into water and oxygen through catalyzation by iodide." The reaction is typically done with potassium iodide, though the iodide ion is the catalyst.

The decomposition of hydrogen peroxide into water and oxygen through catalyzation by iodide. GIF Courtesy of GIPHY
H2O2 actually decomposes slowly, but when we add a catalyst, the reaction speeds up—leading to the "Elephant's Toothpaste" reaction.
Catalysts and Energy
As we saw earlier, a catalyst functions by lowering the Ea (activation energy) for a reaction. Let's take a look at how this works graphically:
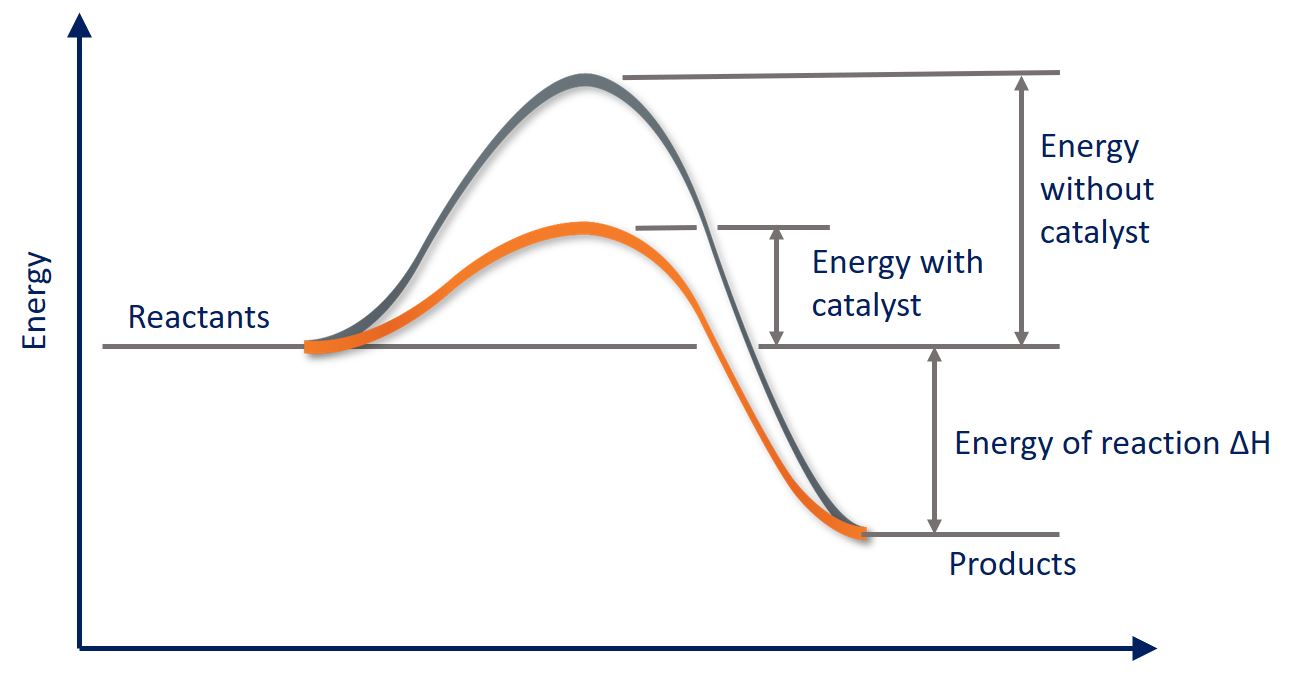
We know that the activation energy is the energy between the reactants and the activated complex (the hump). Then, we see that the addition of a catalyst brings down the curve to lower the activation energy. Catalysts may also split a one-step high Ea into a multi-step, low Ea reaction:
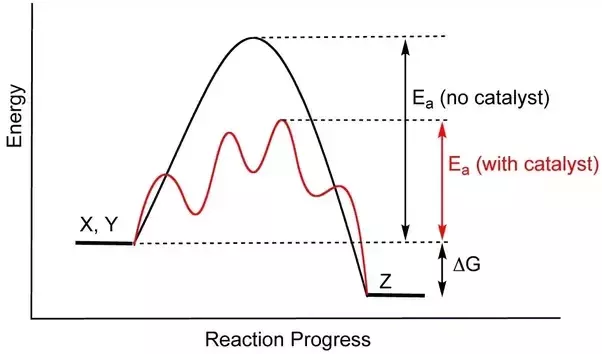
Images Courtesy of Quora
Catalysts and Mechanisms
Although it is less important to know than the thermodynamic effects, catalysts also change the way a mechanism works! We can see this in two mechanisms for the decomposition of H2O2, one being catalyzed and the other with no catalysis.
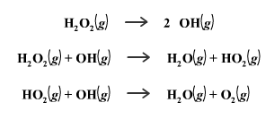
Non-catalyzed Mechanism

Catalyzed Mechanism
The catalyzed mechanism has two steps, whereas the uncatalyzed mechanism has three steps. By eliminating a step, the catalyzed reaction occurs more quickly.
In order for catalysts to increase the rate of a reaction, the addition of the catalyst must increase the number of effective collisions and/or provide a reaction path with a lower activation energy relative to the original reaction. How exactly do catalysts do this?
A common mechanism of catalysts to accelerate reactions is by binding to the reactants. The reactants are either oriented more favorably or react with lower activation energy in the presence of the catalyst, similar to the function of enzymes. However, other catalysts, such as acid-base catalysis, form covalent bonds with reactants whereby a reactant or intermediate either gains or loses a proton. This introduces a new reaction intermediate and new elementary reactions.
Importantly, although the catalyst is frequently consumed by the rate-determining step, the net catalyst concentration remains constant. Therefore, the catalyst is always regenerated in catalysis mechanisms!

© 2024 Fiveable Inc. All rights reserved.