<< Hide Menu
Dalia Savy
Dalia Savy
From the College Board
Develop Your Understanding of this unit
According to the College Board, "This unit explores chemical transformations of matter by building on the physical transformations studied in Unit 3. Chemical changes involve the making➡️⬅️ and breaking⬅️➡️ of chemical bonds. Many properties of a chemical system can be understood using the concepts of varying strengths of chemical bonds and weaker intermolecular interactions. When chemical changes occur, the new substances formed have properties that are distinguishable from the initial substance or substances.
Chemical reactions are the primary means by which transformations in matter occur. Chemical equations are a representation of the rearrangement of atoms that occur during a chemical reaction. In subsequent units, students will explore rates at which chemical changes occur."
Big Idea Questions
- What makes fireworks explode?
- Why is the mass of a raw egg different than a boiled egg?
- What are the processes related to changes in a substance?
AP Chemistry Unit 4: Chemical Reactions
Unit 4 is about all of the chemical reactions behind processes we see every day. 💥
There are so many different types of chemical reactions and with this unit, you begin to understand the mechanics behind each of them. There is quite a bit of math that comes with this unit as well. It also sets up the foundations for future units so it's best to nail this unit before continuing on with the course.
About 7-9% of the AP Chemistry exam is about this unit! 🧠
🧪4.1 Introduction for Reactions
So what happens in chemical reactions? In a simplified definition, specific interactions with molecules result in the rearrangement of atoms to create new molecules.
There are many categories of chemical reactions, but we go over the simple ones in this introductory guide. Synthesis reactions are those that form a complex molecule from two simpler molecules. Decomposition reactions are the opposite: they break down complex molecules into simpler parts.
Combustion reactions are a special type of decomposition reaction where hydrocarbons, molecules made of only carbon and hydrogen, burn in the presence of oxygen. Combustion reactions always follow this format: Hydrocarbon + Oxygen → Carbon Dioxide + Water, and they release a ton of energy.
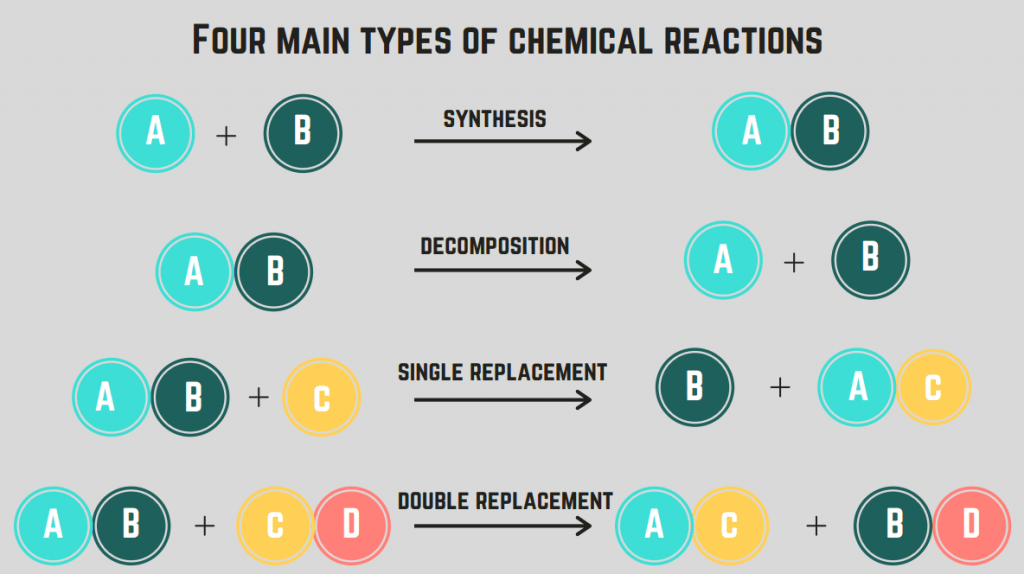
Image Courtesy of Chemtalk
Then, we got replacement reactions! Single replacement reactions involve the replacement of one element in a compound by another element. Double replacement reactions involve the exchange of ions between two compounds to form two new compounds.
🔢4.2 Net Ionic Equations
When dealing with chemical reactions, it is important to understand that, at least in the case of AP Chemistry, the vast majority of them will be taking place in an aqueous solution, that is, dissolved in water. Therefore, we will not be dealing with molecules, but rather their constituent particles that are created when they dissolve.
A net ionic equation is a chemical equation that shows only the species participating in a chemical reaction and omits the spectator ions. Spectator ions are ions that appear on both sides of the arrow in a chemical equation but do not actually participate in the reaction.
In this study guide, you'll learn how to write a net ionic equation using solubility rules.
📊4.3 Representations of Reactions
Just as net ionic equations provide information about interactions with ions in aqueous solutions, chemical reaction equations are a great way to visually track physical changes and chemical reactions. However, before we can examine these equations to observe these changes, we need to learn how to properly set up the equation for a chemical reaction.
The law of conservation of mass is a fundamental principle in chemistry that states that the amount of matter stays constant in a closed system. In short, matter cannot be neither created nor destroyed in thin air. This makes it necessary for us to make sure that our chemical equations are balanced; that is, we need to make sure that the number of elements in the reactants is the same as those in the products, even if the products are different than what we started with. We'll do lots of practice balancing equations so that you're a master at it!
🔬4.4 Physical and Chemical Changes
Chemical equations show the products that the combination of reactants yields, but the equation doesn’t show how the reactants form those products. What we don’t see are the underlying chemical and physical changes that allow molecules to rearrange to change properties or create new substances.
Generally, chemical changes involve intramolecular (literally meaning “inside molecule”) bonds. This includes breaking and/or forming ionic or covalent bonds between elements during a chemical reaction. Physical changes, on the other hand, are usually intermolecular changes (literally meaning “between molecules”), such as phase changes. Some examples are freezing water and cutting paper.
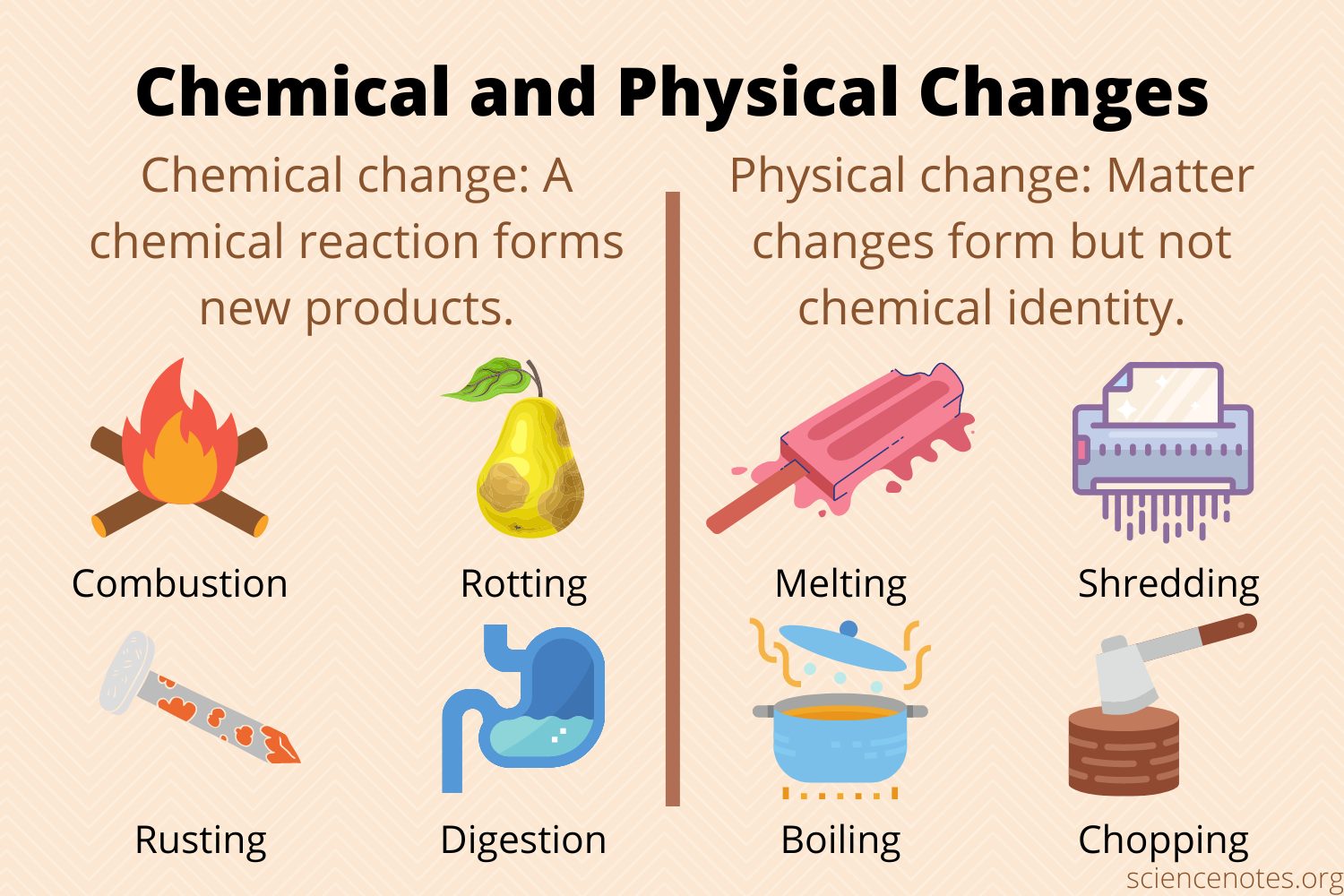
Image Courtesy of Science Notes
⚖️4.5 Stoichiometry
Now, we’ll learn how to quantitatively analyze reactions using stoichiometry! It may seem like a lot of math at first, but once you do more practice, you’ll be more confident and become a stoichiometry master. 👨🏫
Stoichiometry is used specifically to quantify the amount of reactants and products used in a chemical reaction.
📈4.6 Introduction to Titration
Titrations are an experimental method🧪 used to determine the unknown concentration of a chemical solution. The titrant refers to the solution of known concentration, and it is added to the analyte, the solution of unknown concentration.
At some point during the acid-base titration, a color change is observed. This is called the endpoint of a titration. The equivalence point is the point at which the number of moles of titrant added is equal to the number of moles of the analyte.
🔥4.7 Types of Chemical Reactions
At this point in this unit, you've got the basics of chemical reactions and writing chemical equations down! From here, you'll begin to learn about the specifics of three major types of reactions: precipitation reactions, acid-base reactions, and oxidation-reduction reactions.
Acid-base reactions are chemical reactions that involve the transfer of a proton from one molecule to another. Oxidation-reduction reactions, also known as redox reactions, involve the transfer of electrons. Precipitation reactions, though, are completely different. They are those in which two or more soluble reactants combine to form an insoluble product.
🍊4.8 Introduction to Acid-Base Reactions
Okay, so...you know that acid-base reactions involve the transfer of a proton, but what else do you need to know? Acids and bases have many different definitions, but in this course, you'll use the Brønsted-Lowry definitions.
This definition focuses on the transfer of a proton, so acids are proton donors while bases are proton acceptors. What you basically see happen is a hydrogen ion being transferred from a substance, denoted as the acid, to another substance, denoted as the base.
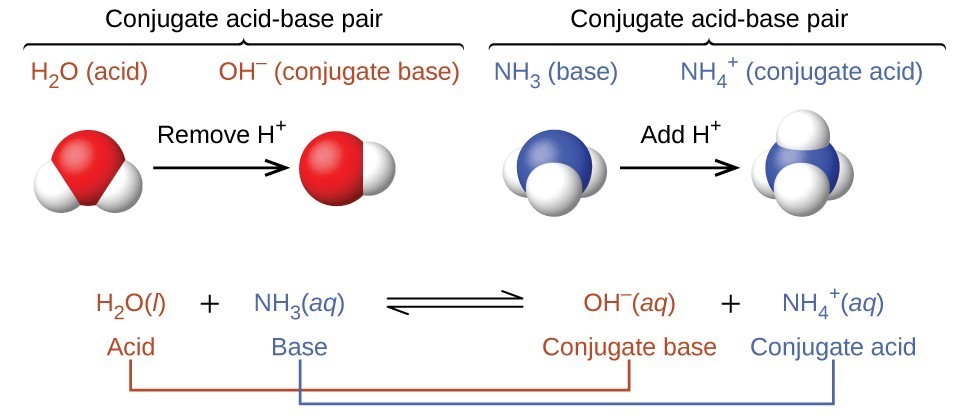
Image Courtesy of Lumen Learning
⚡️4.9 Oxidation-Reduction (Redox) Reactions
Last but not least, redox reactions! These reactions deal with the transferring️ of electrons, which causes molecules to change oxidation states. An oxidation state is a measure of the degree of oxidation of an atom in a chemical compound. It is represented by a positive or negative number that expresses the number of electrons that an atom has gained or lost in a compound relative to its elemental state on the periodic table.
When a molecule loses an electron, it’s oxidized, and its oxidation number increases. When a molecule gains an electron, it’s reduced, and its oxidation number decreases. Electrons travel from the oxidized species to the reduced species.
In this study guide, you'll learn how to assign oxidation numbers to atoms, as well as how to balance redox reactions in both acidic and basic solutions.
📝Unit 4 Key Vocabulary
Physical Change - a change in the form or appearance of a substance, but not its chemical composition.
Chemical Change - change in the chemical composition of a substance.
Synthesis Reactions - reactions in which two or more reactants combine to form a single product.
Decomposition Reactions - reactions in which a single reactant breaks down into two or more products.
Single Replacement Reactions - reactions in which an element in a compound is replaced by another element.
Double Replacement Reactions - reactions in which two elements in different compounds exchange places to form new compounds.
Combustion Reactions - reactions in which a fuel reacts with oxygen to produce heat, light, and various products such as water and carbon dioxide.
Dissociation - the process of a molecule breaking into ions in a solution.
Insoluble - a substance is insoluble if it does not dissolve in a particular solvent.
Spectator Ions - ions that are present in a chemical reaction, but do not participate in the transfer of electrons or protons.
Net Ionic Equation - a chemical equation that shows only the species that are involved in a chemical reaction, and not the spectator ions.
Complete Ionic Equation - a chemical equation that shows all of the ions that are present in a solution, including the spectator ions.
Stoichiometry - the study of the relationships between the reactants and products in a chemical reaction.
Titration - a laboratory technique used to determine the concentration of a solution by adding a known concentration of another solution.
Equivalence point - is the point at which the number of moles of the titrant (the solution being added) is equal to the number of moles of the analyte (the solution being analyzed).
Conjugate Base - the species that is formed when an acid donates a proton.
Conjugate Acid - the species that is formed when a base accepts a proton.
Limiting Reactant - the reactant that is completely used up in a chemical reaction, limiting the amount of product that can be formed.
Excess - a reactant that still exists in solution after the chemical reaction is complete.
Neutralization - the process of an acid and a base reacting to form a salt and water.
Amphiprotic - An amphiprotic substance is a substance that can act as either an acid or a base, depending on the solution it is in.
Redox - stands for reduction-oxidation, and refers to chemical reactions in which the oxidation states of atoms are changed.
Oxidation # - The oxidation number of an atom is a measure of its oxidation state, which is the number of electrons that an atom has gained or lost in a compound relative to its elemental state.

© 2024 Fiveable Inc. All rights reserved.