<< Hide Menu
Dylan Black
Dalia Savy
Anika P
Dylan Black
Dalia Savy
Anika P
From electronic devices📱 to fizzy soda🥤, our world runs on chemical reactions. So what happens in chemical reactions? In a simplified definition, specific interactions with molecules result in the rearrangement of atoms to create new molecules. However, this magic has restrictions, as will be discussed in the next sections.
What is a chemical reaction?
There are two types of changes in chemistry when discussing matter: physical changes and chemical changes.
A physical change is one that changes an object but does not change its chemical structure. For example, you may boil water into steam, but H2O stays H2O💧 during that process. Similarly, shredding paper would be an example of a physical change. Paper may change from a piece to shreds, but it stays "paper"📄.
Changes in the state of matter of a substance or the formation/separation of substances are common physical changes. Nothing changes on a molecular level, rather, the properties of the substance change.
However, chemical changes, as the name implies, end with a brand-new product. There must be some sort of change on a molecular level with chemical changes! For example, if you leave iron out for too long, it rusts. Rust is a chemical reaction between iron (Fe) and oxygen in the air (O2) to form rust (iron oxide, Fe2O3).
Another example of a chemical change is mixing baking soda (NaHCO3) with vinegar (CH3COOH) to form carbon dioxide and other products. Chemical changes are the focus of AP Chemistry and are described using chemical reactions. The following are observations that are evidence of a chemical change, and therefore, a chemical reaction taking place:
- Heat or light - combustion reactions, as we'll discuss below, are evident with fire.
- Formation of gas (and maybe an odor) - you may see bubbles.
- Precipitation - this refers to the formation of a solid when two solutions mix
- Color change - using indicators, or chemicals that change color based on pH.
How are chemical reactions represented?
A chemical reaction can be represented using a chemical equation. A chemical equation is a written representation of the substances involved in a chemical reaction and the changes they undergo. Here is how to read a chemical equation:
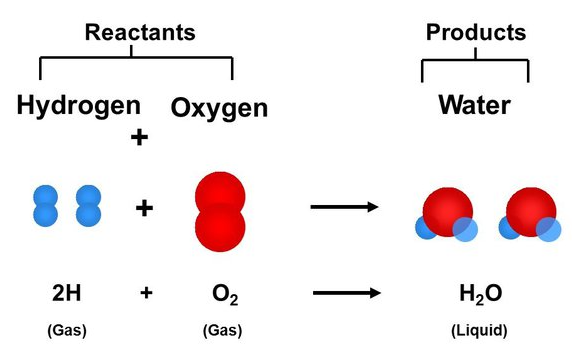
Image Courtesy of Quora
- The reactants, or the substances that are reacting, are listed on the left side of the equation.
- The products, or the substances that are produced, are listed on the right side of the equation.
- The arrow (→) indicates the direction of the reaction, from left to right.
- The coefficients, or the numbers in front of the reactant and product formulas, indicate the relative amounts of each substance involved in the reaction. For example, in the reaction 2H2 + O2 -> 2H2O, the coefficient "2" in front of H2O indicates that two molecules of water are produced.
- The formulas of the reactants and products represent the chemical compositions of the substances involved in the reaction. The formulas are made up of symbols for the elements present in the substances, with subscripts indicating the number of atoms of each element. For example, the formula H2O represents a molecule of water, which is made up of two atoms of hydrogen and one atom of oxygen.
There are five main types of reactions: synthesis, decomposition, combustion, single replacement, and double replacement. While we will go over these in later sections, let’s do a brief overview of each type:
Synthesis Reactions
Synthesis reactions are the easiest type to understand. Essentially, a synthesis reaction sees two reactants fuse to form products in the form A + B → AB, where A, B, and AB are arbitrary.
An example of a synthesis reaction is 2Na + Cl2 → 2NaCl, as it sees two reactants, Na and Cl2, become one product: NaCl. The synthesis of water from hydrogen and oxygen is another example of a synthesis reaction: 2H2 + O2 → 2H2O.

The Synthesis of Sodium Chloride from Sodium Metal and Chlorine Gas
Decomposition Reactions
Decomposition reactions are the exact opposite: one reactant decomposes into two or more products. This can be represented by the general equation: AB → A + B.
For example, hydrogen peroxide decomposes into hydrogen gas and water in the reaction 2H2O2 → 2H2O + H2. You can also reverse the synthesis of water in the last section to represent the decomposition of water: 2H2O → 2H2 + O2.

The Elephant's Toothpaste: A violent reaction that is caused by a speeding up of the decomposition of hydrogen peroxide using a catalyst
Combustion Reactions
A combustion reaction is a special type of decomposition reaction involving organic molecules, which are molecules that are carbon-based. A special class of organic molecules is called hydrocarbons which are only made up of hydrogen and carbon molecules.
When in the presence of heat and oxygen, hydrocarbons combust or burn which is really just the energy being released from a chemical reaction! Therefore, a general combustion reaction sees a hydrocarbon being combusted into carbon dioxide and water in the presence of oxygen.
Combustion reactions always follow this format: Hydrocarbon + Oxygen → Carbon Dioxide + Water. For example, CH4 + 2O2 → CO2 + 2H2O is the combustion of methane (CH4). This definition of combustion is why oxygen is necessary for fires to start.
The Methane Molecule - Image Courtesy of world of molecules
Single Replacement Reactions
Single replacement reactions occur when you have a compound reacting with an element. The general form is AB + C → AC + B. The most common form of a single replacement reaction is one called a redox reaction, where electrons are transferred between atoms. An example is 3Mg + 2AlCl3 → 3MgCl2 + 2Al.
Single = one, replacement = a switch. These reactions involve the replacement of one element in a compound by another element. Only one switch takes place.
Double Replacement Reactions
The most common type of reaction is a double replacement reaction, with the general form AB + CD → AD + CB.
For example, if you mix an acid and a base, you get a reaction that forms water and some type of salt. A formula example would be HCl + NaOH → NaCl + H2O. These reactions are the majority of the reactions you'll see in AP Chemistry, so get used to them!
Double = two, replacement = switch. These reactions involve the exchange of ions between two compounds to form two new compounds. Here, two switches take place.
Practice Questions
Identify what type of reaction each of the following are:
- Zn + 2HCl → ZnCl2 + H2
- 2C8H18 + 25O2 → 16CO2 + 18H2O
- 2H2O → 2H2 + O2
- AgNO3 + NaCl → AgCl + NaNO3
- 2Ni2O3 → 4Ni + 3O2
- 2Na + Cl2 → 2NaCl
- Cl2 + 2NaBr → 2NaCl + Br2
- BaCl2 + Na2SO4 → BaSO4 + 2 NaCl
- C3H8 + 5O2 → 3CO2 + 4H2O
Answers
- Single Replacement - Zinc is "switching places" with hydrogen to form zinc chloride and hydrogen gas.
- Combustion - You can see the general formula of a combustion reaction of a hydrocarbon combusting in the presence of oxygen to form carbon dioxide and water.
- Decomposition - Here, you can see the division of one compound into simpler molecules.
- Double Replacement - This reaction involves the exchange of two ions to form two new compounds: silver chloride and sodium nitrate.
- Decomposition - This is another example of a complex compound being divided, or decomposed, into its simpler components.
- Synthesis - This is the opposite of the last question! You have two elements coming together to form a compound: sodium chloride.
- Single Replacement - In this single replacement reaction, we can see chloride replacing bromide to form sodium chloride and bromide molecules.
- Double Replacement - Another very common reaction where two ions exchange places!
- Combustion - Remember, whenever you see a hydrocarbon coming together with oxygen, it will combust into carbon dioxide and water. These reactions are just the basics. Once we dive deeper into the unit, we'll go over three important reactions:
- Precipitation Reactions
- Acid-Base Reactions
- Oxidation-Reduction Reactions 🎥 Watch AP Chemistry teacher Wes Winter define the evidence for a reaction and identify the different types of reactions.

© 2024 Fiveable Inc. All rights reserved.