<< Hide Menu
Hope Arnett
Dalia Savy
Hope Arnett
Dalia Savy
What Are Titrations?
Titrations are an experimental method🧪 used to determine the unknown concentration of a chemical solution. There are two key substances in a titration: the titrant and the analyte.
- The titrant is a solution of known concentration that is used to determine the concentration of the unknown solution in a titration. It is usually added to the unknown solution using a burette, which you can see is a long, narrow tube with a stopcock. This allows the chemist to precisely control the volume of titrant they add to the unknown solution.
- The analyte is the unknown solution whose concentration is determined in a titration. It is typically in the Erlenmeyer flask below the burette. You may sometimes see the analyte referred to as the titrand. The image below shows a set-up of the apparatus chemists use to conduct titrations.
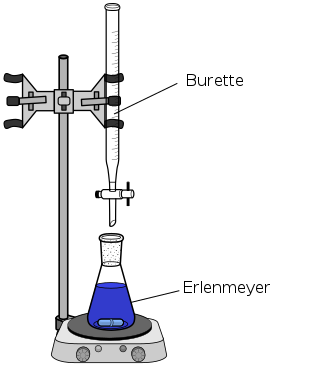
Types of Titrations
There are several types of titrations in chemistry, but this course specifically focuses on acid-base titrations. We'll focus on them in this study guide, but here is a quick rundown of the others as well:
- Acid-base titrations: Acid-base titrations are used to determine the concentration of an acid or a base in a solution. The endpoint in an acid-base titration is usually indicated by a pH change, which can be detected using a pH meter or an indicator solution.
- Redox titrations: Redox titrations are used to determine the concentration of an oxidizing or reducing agent in a solution. The endpoint in a redox titration is usually indicated by a color change, which can be detected using an indicator solution or an electronic meter.
- Precipitation titrations: Precipitation titrations are used to determine the concentration of a substance that can form a precipitate with another substance. The endpoint in a precipitation titration is usually indicated by the formation of a precipitate, which can be detected visually or by measuring the light transmittance of the solution.
- Complexation titrations: Complexation titrations are used to determine the concentration of a complexing agent in a solution. The endpoint in a complexation titration is usually indicated by a change in the color or absorption of the solution, which can be detected using an indicator solution or a spectrophotometer. You're probably wondering what an endpoint is, but we'll go over that shortly!
Acid-Base Titrations
Don't worry about the other types of titrations! Let's dig deeper into acid-base titrations.
The titrant in the burette is typically a strong acid or base (and remember, we know its concentration 🍊). The analyte of unknown concentration is in the Erlenmeyer flask beneath the burette and is usually a weak acid or base.
Both the burette and Erlenmeyer flask have tick marks for measurement, so we know the initial volumes of both solutions and can do the necessary math to solve for the molarity of the analyte. Remember, molarity (M) is a measure of concentration and can be calculated by dividing the moles of the substance by its volume in liters. This is why we need to keep a record of how much solution is used.
Along with the weak acid or base, the Erlenmeyer has a drop or two of an indicator. There are different types of indicators, and each indicator changes the solution’s color when it is within a certain pH range.
Carrying out a Titration
When you a preparing a titration, you:
- Fill the burette with the titrant of known concentration. Note the concentration and volume of the titrant in the burette before starting the titration.
- Measure out the desired volume of the analyte and place it in the Erlenmeyer flask.
- Add a few drops of indicator to the analyte in the Erlenmeyer flask. Now, you're ready to start the titration! From here, you gradually add the titrant from the burette into the flask, stirring constantly, until the indicator changes the color of the solution. There are two points during a titration that are kept note of:
- Equivalence point: The equivalence point is the point at which the number of moles of titrant added is equal to the number of moles of the analyte. At the equivalence point, the reactants are completely consumed and the products are present in stoichiometric amounts. It indicates the end of the reaction.
- Endpoint: The endpoint is the point at which there is a visible change in the Erlenmeyer flask, such as a color change or a pH change. When the correct indicator is used, the endpoint of the titration indicated by a color change occurs at the equivalence point where the moles of titrant = the moles of the analyte.
A common indicator is phenolphthalein, which makes the solution light-pink at the equivalence point. If the analyte is a weak acid and the titrant is a strong base, the pH of the analyte is less than 7 before the titration begins. At this point, the phenolphthalein makes the solution colorless. However, as the base is added, the solution's pH increases. At the endpoint, once a certain amount of strong base is added, the solution changes color.
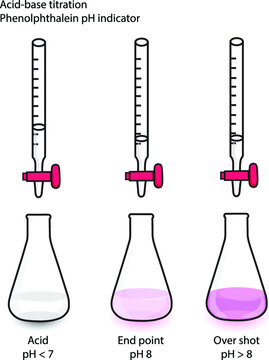
Image Courtesy of Adobe Stock
Graphical Representations of Titrations
A titration curve can be produced after completing a titration. A titration curve is a graphical representation of the relationship between the volume of titrant added and the corresponding pH of the analyte.
Below is a sample graph of the curve:
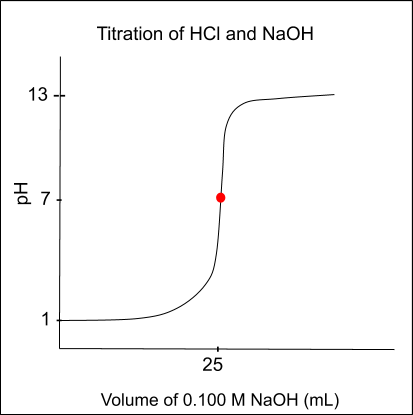
There are three main regions on a titration curve:
- The linear region: The linear region is the portion of the curve where the pH of the analyte is pretty constant as more titrant is being added to the solution.
- The inflection point: The inflection point is the point on the curve where the slope changes. It indicates the equivalence point, which is the point at which the number of moles of titrant added is equal to the number of moles of the analyte. In the graph above, it is the red dot at a pH of 7.
- The endpoint: The endpoint is the point on the curve where the chemical reaction between the titrant and the analyte is complete and is indicated by a color change. Remember, you typically want the endpoint and equivalence point to be the same.
Simple Titration Calculations
Since the equivalence point is when the number of moles of acid and base are equal, we can make an equation using molarity (moles/L) and volume:
moles of acid = moles of base
Since moles = molarity x liters, we can say that the molarity of the titrant times the volume of the titrant equals the molarity of the analyte times the volume of the analyte or MaVa = MbVb.
We know the titrant’s concentration going into the titration, so we can use that for Ma. Va is the amount of titrant added to the Erlenmeyer flask. Vb is the analyte’s initial volume. We can plug in these values and then solve for Mb.
** It’s important to note that if the mole ratio of the acid and base is NOT 1:1, then you would need to multiply the appropriate sides by their mole ratio. For example, if an acid and base have a mole ratio of 1:2, then this would be the equation: MaVa = 2MbVb**
Titration Calculations Example
The following question is from the Advanced Placement YT Channel.
A solution of vinegar contains an unknown amount of acetic acid, HC₂H₃O₂. A 25.0 mL sample of vinegar is titrated with 0.650 M NaOH according to the chemical reaction below. If it requires 32.04 mL of the titrant to reach the equivalence point, what is the concentration of HC₂H₃O₂ in the vinegar?
HC₂H₃O₂ (aq) + NaOH (aq) → H₂O (l) + NaC₂H₃O₂ (aq)
This requires the simple use of MaVa = MbVb. Since the mole ratio is 1:1, we can just plug in and solve!🎉
(Ma)(25.0 mL) =(0.650 M)(32.04 mL)
Ma = 0.833 M
Acids and Bases
Acid-base reaction equations illustrate what is happening during titrations. In the Brønsted-Lowry definition, acids are proton donors and bases are proton acceptors. The species that donated the proton becomes the acid’s conjugate base. Similarly, the species that accepted the proton is now the conjugate acid.
By looking at chemical equations, we can identify which species are the acid, base, conjugate acid, and conjugate base. Check out the example below:
HCl (aq) + NaOH (aq) → NaCl (aq) + H₂O (l)
Take a look at the reactants and the products. Notice that this is a double replacement reaction. The reactants’ ions split up, and the H⁺ from HCl joined with OH⁻ to make water. The remaining ions are then combined.
Since HCl gave up its proton, and hydroxide from NaOH accepted it, HCl is the acid, and NaOH is the base. Their corresponding products are their conjugates.
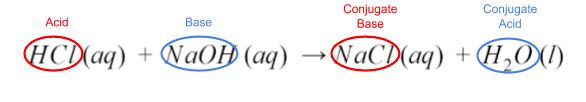
Additionally, the equation illustrates another property of acid-base reactions. When reacted completely, the acid and base neutralize each other, creating a salt and water. Water is a special case--it’s amphiprotic. This is a fancy term to describe how water can be both a proton donor and a proton acceptor. In other words, water can act as either an acid or a base.
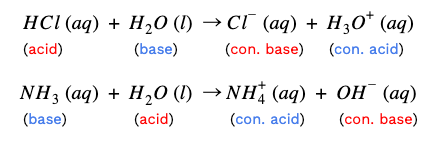
In the first example, HCl donated a proton to water, so water acts like a base here. However, in the second equation, NaOH accepts a proton from water, so water acts like an acid. Finally, another note: ionizing acids and bases in water reveal the strengths of their conjugate pairs.
Shortcut
There is a quick way to figure out the conjugate acid and base! First, find the pairs!
Let's use NH₃ + H₂O → NH₄⁺ + OH⁻ as the example.
NH₃ and NH₄⁺ are the first pair.
H₂O and OH⁻ are the second pair.
But how do we know which one is the acid and which one is the base in each pair?💭
Simple! The compound with the extra hydrogen is the acid. Therefore, NH₄⁺ is the conjugate acid and H₂O is the acid.
Review Activity
Identify the acid and base and their conjugates.
- H₂SO₄ (aq) + CH₃NH₂ (aq) → CH₃NH₃⁺ (aq) + HSO₄⁻ (aq)
- NH₃ (aq) + HNO₂ (aq) → NH₄⁺ (aq) + NO₂⁻ (aq) Answers:
🎥 Watch Dylan Black discuss titrations and how to determine the molarity of a solution at the equivalence point.

© 2024 Fiveable Inc. All rights reserved.