<< Hide Menu
Dalia Savy
Kanya Shah
Dalia Savy
Kanya Shah
Intermolecular forces (IMFs) are the attractive or repulsive forces between entire molecules due to differences in charge. Many students confuse IMFs with intramolecular forces, which were the center of the last unit. Try to remember the following:
- Intermolecular forces - forces that hold molecules together.- Types: London dispersion forces, dipole-dipole forces, hydrogen bonds, and ion-dipole forces
- Intramolecular forces - forces within a molecule that hold atoms together.- Types: covalent bonds, metallic bonds, ionic bonds 🤔 One way to remember the difference is that "inter" means between, while "intra" means within.
IMFs are much weaker than intramolecular forces. This is due to the simultaneous attraction for electrons that exist between two nuclei within a molecule!
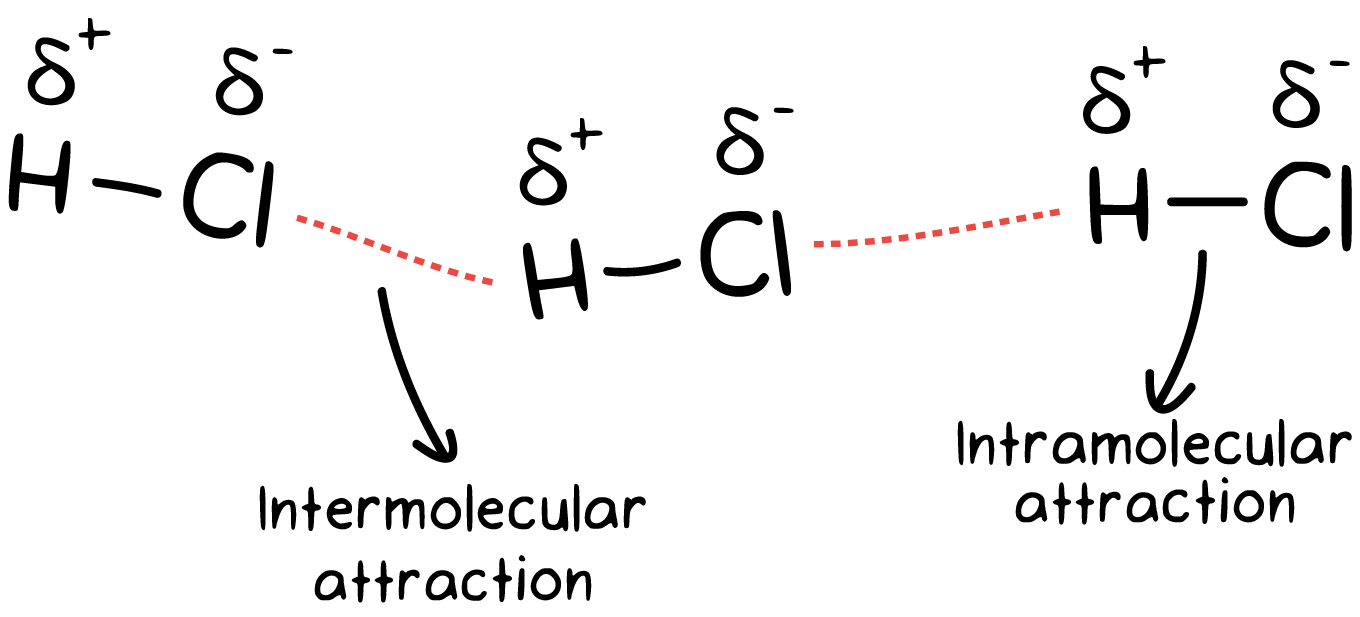
Image Courtesy of Khan Academy
You can also think of IMFs as weaker because they exist over a larger distance. Remember that Coulomb's law states that the closer the two particles are, the stronger the attraction.
In this study guide, we'll learn about four types of intermolecular forces: London dispersion forces, dipole-dipole forces, hydrogen bonding, and ion-dipole forces.
London Dispersion Forces
London Dispersion Forces (LDFs) are the weakest type of IMFs and occur in all molecular samples. They are the only types of forces to exist between two non-polar molecules and noble gases (solid or liquid form).
On the AP Exam, if you are asked to list the types of IMFs being seen in a certain molecule, London dispersion forces will always be an answer. They can easily be forgotten so make sure they always show up in your answer (along with any other forces present)!
At any given time, one nonpolar molecule might have more electrons on one side than the other side, making it polar. For that instant, the molecule would have a partial negative side and a partial positive side and it creates a temporary dipole.
The molecule with the temporary dipole then induces a dipole on its neighboring molecules, creating a London dispersion force between the partial negative side of one molecule and the partial positive side of another molecule.
In other words, London dispersion forces are a result of Coulombic interactions between temporary and fluctuating dipoles.
Here is a quick visual to sum up this idea of an LDF:
The strength of LDFs increases as the size of a molecule increases. This is because more electrons yield a stronger instantaneous dipole due to a more polarizable electron cloud. Polarizability is the ease at which an electron cloud could be distorted to give a dipole charge distribution.
The concept discussed in the last paragraph is a very important piece of information that is typically asked on the AP exam. The more electrons there are, the greater the size of the electron cloud, and the more polarizable it is! This makes it more likely for there to be a temporary dipole.
Dipole-Dipole Interactions
Dipole-dipole attractions occur between the opposite partial charges that exist on opposite ends of a dipole. While LDFs are present due to temporary dipoles, dipole-dipole forces result from permanent dipoles. As a result, dipole-dipole attractions only occur in a sample of polar molecules and are slightly stronger than LDFs.
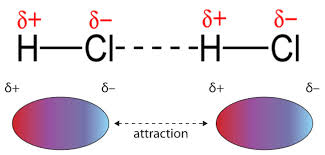
The dipoles in HCl lead to a positive side and a negative side that are attracted to each other.
Image Courtesy of EMedicalPrep As you decrease the distance between the two dipoles, you strengthen the dipole-dipole interaction. This relates back to Coulomb's law. Keep in mind, since these involve polar molecules, the element with the higher electronegativity (in this case Cl-) has a partial negative charge (δ−). This also means that the more polar the molecules, the greater the dipole-dipole attraction. The stronger the attraction, the higher the melting and boiling points of the sample.
AP Question - LDFs + Dipole-Dipole
The following question is from the 2018 AP Chemistry examination posted online by College Board.
The table above gives the molecular structures and boiling points for the compounds CS2 and COS. In terms of the types and relative strengths of all intermolecular forces in each compound, explain why the boiling point of CS2 (l) is higher than that of COS (l).
Sample Response: CS2 and COS both have London Dispersion Forces, but since COS is a polar molecule, it also exhibits dipole-dipole forces. However, the London Dispersion Forces in CS2 are so strong that they overpower the strength of both the LDFs and the dipole-dipole forces in COS. Therefore, CS2 has a higher boiling point.
🧠 Keep in mind that even though LDFs are the weakest forces, they can overpower dipole-dipole forces. It all depends on the size of the molecule; when there are many London dispersion forces present, they can be stronger.
You will not be expected to predict when the LDFs are stronger than the dipole-dipole forces present, but you will rather be given information that tells you this. In this question, you are given the boiling points to tell you that the intermolecular forces present in CS2 are stronger than those in COS.
Hydrogen Bonding
Hydrogen bonding is really everyone's favorite! It's the easiest to identify and the strongest in pure substances, so they really stick in your mind throughout the whole year😊.
Hydrogen bonding (which is NOT a bond) is actually an unusually strong dipole-dipole attraction and only occurs when hydrogen is directly bonded to F, O, or N in a molecule. It occurs between these molecules because of their high electronegativity difference and small sizes, which leads to really really strong attractions.
Remember "Hydrogen bonding is FON," or fun, and that it only occurs in polar molecules!
The hydrogen bonded to the F, O, or N is partially positively charged and is attracted to the neighboring unshared electrons on the F, O, or N.
Since these attractions are super strong, the boiling points of molecules with hydrogen bonds are very high! An example of a molecule that has hydrogen bonding is water, which is part of why it takes so long for water to boil. The amount of energy needed to break the intermolecular forces between water molecules when boiling is relatively high. Hydrogen bonding is also seen in DNA🧬, and you know how strong those attractions need to be!
Let's review the misconception once more before moving on:
On the AP Exam, you may be asked to draw a molecule with the proper orientation and create hydrogen bonding. Make sure you don't confuse the difference between intermolecular and intramolecular. Remember, intermolecular is in between two molecules. Fiveable's here to help! 🎉
🎥 Watch Jacob Jeffries review polarity and discuss dipole-dipole forces, hydrogen bonding, and London dispersion forces. He reviews their significance to physical properties, along with applications in biomolecules.
Ion-Dipole Forces
Ion-dipole attractions only occur in a mixture of an ionic compound and polar molecules. These attractions occur when ions (cations or anions) are attracted to the positive or negative end of a dipole.
Overall, this IMF is stronger than dipole-dipole and H-Bonding.
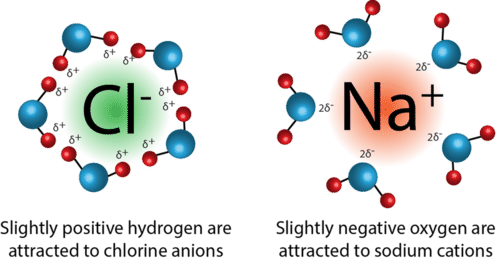
Image Courtesy of ck12.org
An example of ion-dipole forces is when NaCl is dissolved in a sample of water. Once the NaCl dissolves, it separates into its positive sodium cation and the respective negative chloride anion. From here, the positive end of the water molecule (hydrogen) is attracted to the negative chloride anion, while the negative end of the water molecule (oxygen) is attracted to the positive sodium cation.
Let's break it down further: when we take a look at a water molecule, oxygen has a partial negative charge. This is due to its high electronegativity, and it more strongly attracts electrons toward it. This unequal distribution of electrons results in partial positive and negative charges, or a dipole. The charges produced in this dipole can then attract the ions produced by the dissolution of NaCl.
Ion-Ion Attractions
Ion-Ion attractions occur in a sample of ionic compounds. This is the strongest type of attraction because there aren’t partial charges, but instead full charges on the ions.
These attractions hold the ions together in a solid crystal lattice, giving rise to the high melting and boiling points of ionic compounds. Think about the formation of NaCl, where the sodium atom donates one of its electrons to the chloride atom, forming a sodium cation and chloride anion. The positive sodium ion is attracted to the negative chloride anion through ion-ion attractions.
These forces can be confused with ion-dipole interactions, but try to think about each example given. Ion-dipole interactions are present when NaCl dissolves in water, but ion-ion interactions are present within a solid sample of NaCl itself.
👉 Not sure what a crystal lattice is? Check out our study guide about the structure of ionic solids.
Summary of IMFs
You may be wondering how we can determine the dominant IMF - well it’s quite easy with this simple diagram:
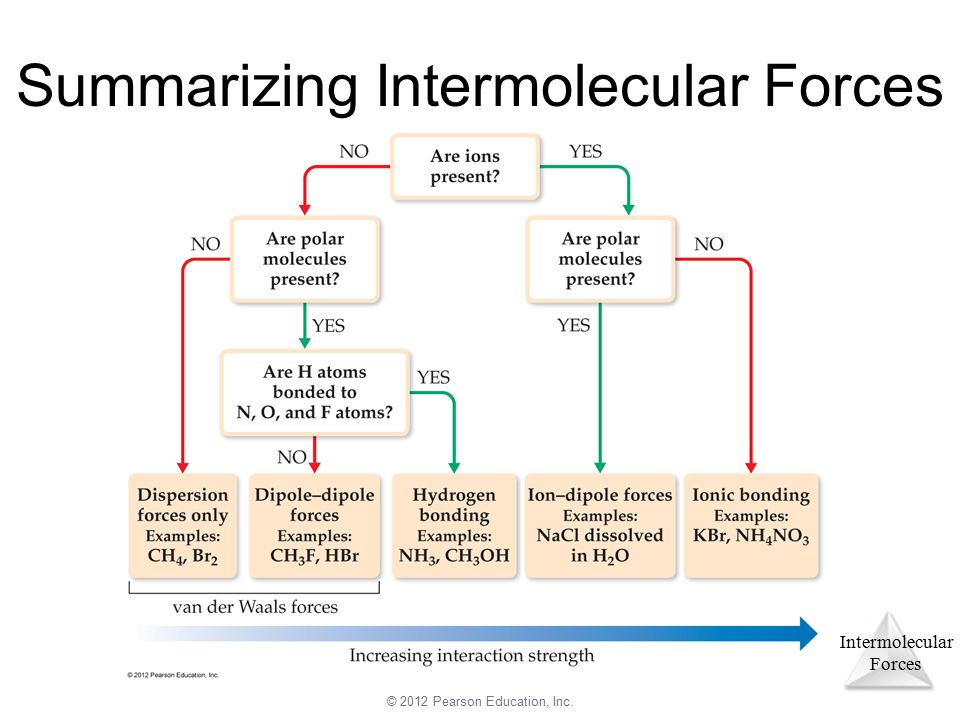
Image Courtesy of Pearson Education
🎥Watch AP Chemistry teacher Wes Winter discuss the importance of intermolecular forces and how the strength of these forces affects the bulk scale properties.

© 2024 Fiveable Inc. All rights reserved.