<< Hide Menu
Unit 2 Overview: Molecular and Ionic Compound Structure and Properties
7 min read•june 18, 2024
Dalia Savy
Dalia Savy
AP Chemistry Unit 2: Molecular & Ionic Compounds
Welcome to Unit 2 of AP Chemistry! Now that we've learned about atomic structure and the basics in unit one, we can build upon this new knowledge by discussing molecular and ionic Bonding. While the last unit went in-depth about the structure of the atom and electrons, this unit will discuss, at large, the ins and outs of molecular and ionic compounds - compounds with more than one atom or ion bonded together.
Throughout the unit, we will cover:
- the different types of bonds
- how electron orbitals relate to covalent bonding
- how chemists represent different kinds of bonds using: - Lewis Dot Diagrams- VSEPR Theory
According to the College Board, "In Unit 2, students apply their knowledge of atomic structure at the particulate level and connect it to the macroscopic properties of a substance. The structure and arrangement of atoms, ions, or molecules, as well as the forces between them, can determine both the chemical and physical properties of materials. These forces, called chemical bonds, are distinct from typical intermolecular interactions." Let's get started!
In AP Chemistry, unit two accounts for 7-9% of the material you will be tested on.
Unit Two's Big Picture
The overarching question of unit two is "How are compounds arranged?". Bonding is all about potential energy. When atoms and ions bond to form compounds, they decrease the potential energy between them and create different compounds. Decreasing potential energy increases stability which is one of the biggest goals of atoms in chemistry.
🔗2.1 Types of Chemical Bonds
The type of compound that will be formed depends on the atoms that make it up. This key topic discusses two of the three major bonds in chemistry that you will learn about: ionic bonds and covalent bonds. Ionic compounds are made up of ionic bonds, whereas molecular compounds are made up of covalent bonds.
Ionic compounds utilize both a metal and a nonmetal and make up ionic bonds. The nonmental will often "steal" an electron from the metal, usually an alkali or alkaline earth metal (in groups one and two on the periodic table, respectively). The metal then becomes a positively charged ion (a cation), and the nonmetal becomes a negatively charged ion (an anion). They are attracted to each other by electrostatic forces explained by Coulomb's law. For example, electrons are directly transferred from sodium to chlorine to form the salt NaCl, where the sodium cation and chloride anion are joined by an ionic bond.
When atoms "share" electrons among themselves, they are connected via covalent bonds. We'll see that this sharing occurs via overlapping orbitals. For example, two hydrogen atoms and an oxygen atom may react to form H2O, otherwise known as the molecular compound water.
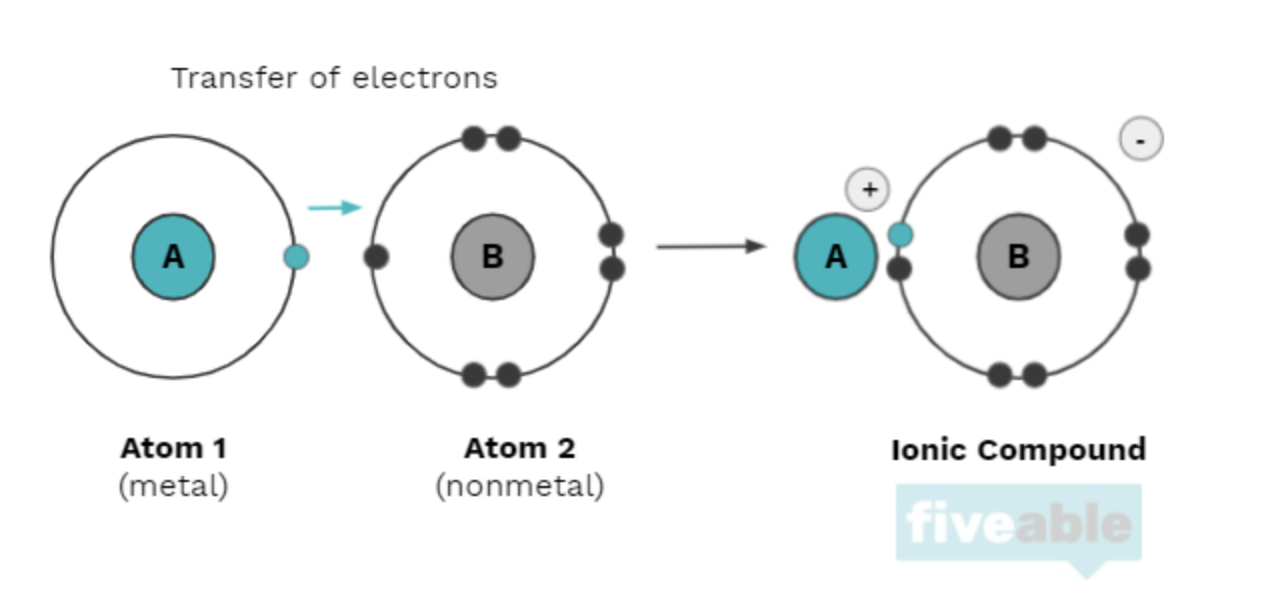
🧲2.2 Intramolecular Force and Potential Energy
You'll also learn the connection between bonding and intramolecular forces, that is, forces that occur between atoms within a molecule. Bonds occur to minimize the potential energy between atoms. When atoms are too close, there is repulsion; when they are far apart, there's no minimal potential energy between them, which you can see in the following graph:
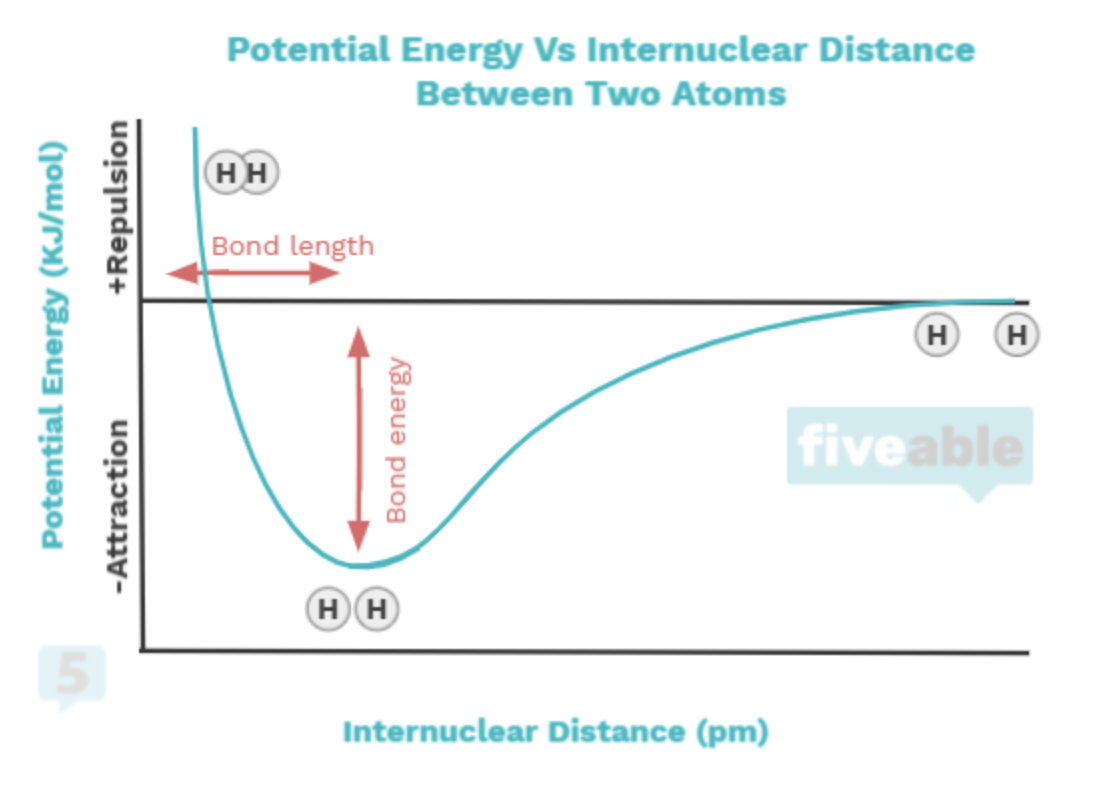
🧂2.3 Structure of Ionic Solids
From here, you'll start to learn about the structure and properties of ionic solids, such as salt (NaCl). Ionic solids are made up of ions that are arranged in a crystal lattice, or a 3-D array. The lattice structure can be explained by the strong electrostatic forces that arise between cations and anions because of their opposing charges. Coulomb's law describes these exact forces, telling us that the electrostatic force between a cation and an anion is directly proportional to their charges and inversely proportional to the distance between them.
🪙2.4 Structure of Metals and Alloys
The third major type of bonds is metallic bonds! Metallic bonds form within metals and alloys in which electrons are delocalized in an "electron sea." The delocalization of electrons in metals allows metals to be very good conductors of heat and electricity!
Alloys can be formed when two or more elements, where at least one is a metal, are in their liquid form being mixed together. There are two types of alloys you will focus on:
- Interstitial alloys - smaller atoms fill the interstitial spaces between larger atoms (ex. steel)
- Substitutional alloys - atoms of one element substitute atoms of another element of similar size (ex. brass)
👀 2.5 Lewis Diagrams
Along with the types of bonds, you'll also learn how chemists theorize about why bonds happen and how they model molecules. Molecules are displayed using Lewis dot diagrams, which show elements, bonds, and valence electrons.
These structures help simplify what a molecule is and what atoms connect to which. While it may be obvious in something like SO (it's pretty to see that this will involve S bonded directly to O), it isn't as evident in a molecule like C2H5OH, so Lewis Structures make it easier.

🎨 2.6 Resonance and Formal Charge
Lewis structures aren't always perfect, which leads to chemists using the concepts of formal charge and resonance to adjust. Resonance is a phenomenon in which a molecule has multiple optimal Lewis structures. This often occurs when there is a double bond in a molecule that can be swapped around without creating unstable formal charges. For example, the nitrate ion exhibits resonance:

In reality, the double bond is delocalized and shared among the three single bonds. Along with resonance, chemists also observe formal charges within Lewis structures. Formal charge describes the charge felt by an atom within a molecule and it can be assigned to each individual atom.
💥2.7 VSEPR and Bond Hybridization
Chemists have also developed theories like VSEPR to explain molecules and their 3-D geometry. With the VSEPR theory in mind, we can name the shape of a molecule and its hybridization.
Hybridization is the idea that atomic orbitals fuse to form newly hybridized orbitals, which in turn, influences molecular geometry and bonding properties.
AP Chemistry Unit 2 Key Vocabulary
QUICK TIP: You will typically not be asked about the definition of these terms directly. You rather will be expected to apply your knowledge of these terms and justify your claims with evidence.
Molecules - a group of atoms bonded together, usually in a fixed ratio and arrangement, to form a stable, discrete chemical structure.
Covalent bond - a chemical bond that involves the sharing of electrons between atoms.
Polar covalent bond - a chemical bond formed between two atoms in which the atoms share electrons unevenly.
Nonpolar covalent bond - a chemical bond between two atoms in which the electrons are shared equally.
Ionic bond - a type of chemical bond that occurs between two atoms or ions when one atom donates one or more electrons to another atom to form a positive and negative ion pair
Coulomb's law - fundamental law that describes the electrostatic force between a cation and an anion in an ionic bond.
Crystal lattice - a regularly repeating three-dimensional arrangement of atoms or molecules in a solid.
Intramolecular forces - the forces that hold atoms together within a molecule.
Cation - a positive ion that has lost one or more electrons.
Anion - a negative ion that has gained one or more electrons.
Delocalization - the spreading out of electrons over a large area (mobile electrons).
Lattice energy - the energy released when ions bond to form an ionic solid.
Metallic bond - a type of chemical bond that occurs between metal atoms and is characterized by the sharing of valence electrons among the metal atoms, resulting in a sea of electrons.
Interstitial alloys - alloys in which the atoms of one element are incorporated into the spaces between the atoms of another element.
Substitutional alloys - alloys in which atoms of one element are substituted for atoms of another element in a regular pattern.
Lewis structures - diagrams that show the arrangement of atoms and valence electrons in a molecule.
Octet rule - states that atoms tend to gain, lose, or share electrons in order to have eight electrons in their outermost energy level, which gives them a stable octet.
Resonance - the ability of a molecule to be described by two or more Lewis structures, with the actual structure being a hybrid of these structures.
Formal charge - the difference between the number of valence electrons an atom has in its neutral state and the number of electrons it is assigned in a Lewis structure.
Molecular Geometry - the three-dimensional arrangement of atoms in a molecule.
Valence Shell Electron Pair Repulsion (VSEPR) - a model that predicts the shape of a molecule based on the repulsion between the electron pairs in the valence shell of the central atom.
Hybridization - the process of mixing atomic orbitals to form new, hybrid orbitals with different shapes and energies.

© 2024 Fiveable Inc. All rights reserved.