<< Hide Menu
AP Chemistry: Electron Shells, Sub-shells, and Orbitals
2 min read•july 11, 2024
Dylan Black
Dylan Black
AP Chemistry spends a lot of time talking about electrons. Where are electrons? How are they configured? How do electrons behave? These questions and more are all ones that AP Chemistry students will encounter.
🎥Live Stream Replay: Introduction to AP Chemistry
🎥Live Stream Replay: AP Chemistry Essentials
What are electrons?
💎💎Electrons are the negatively charged subatomic particles in an atom and are located outside of the nucleus in various energy levels, called shells. These shells are then divided into subshells, which in turn have their own orbitals.
🎥Live Stream Replay: Atomic Structure and Mass Spectroscopy
The Tier List:
- Energy Levels contain subshells- Subshells contain orbitals
🎥Live Stream Replay: Electron Configurations
Subshells and Orbitals
💎💎Subshells are a grouping within a shell, each carrying slightly different energies. The subshells are denoted by the symbols s,p,d, and f.
Subshells are made up of individual orbitals, each of which can contain 2 electrons. So for example, the p subshell has 3 orbitals, each of which can contain 2 electrons.
Here's a table:
Many students mix up subshells and orbitals, so be sure to know the difference! Another representation can be as an energy diagram:
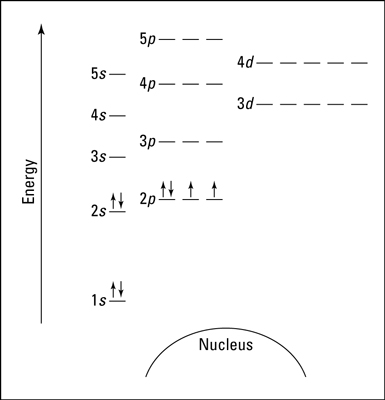
Here, each energy level is notated with a number (1, 2, 3, etc.), each subshell by a letter (s, p, d), and each orbital is represented by a line with electrons being the arrows.
Writing Electron Configurations
An electron-configuration involves simply the number of electrons in the atom. The atom fills orbitals with electrons following this following diagram:
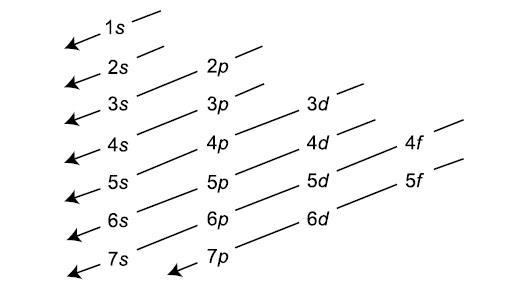
Each s subshell holds 2 electrons, each p subshell holds 6 electrons, each d subshell holds 10 electrons, and each f subshell holds 14 electrons.
Example:
Let's find the electron configuration of sodium (Na). Looking at the periodic table, we can count to find the electron configuration:
We start with the 1s orbital and follow the above chart:
Continuing with this process, we find that the electron configuration of sodium is: 1s2 2s2 2p6 3s1.
🎥Live Stream Replay: Valence Electrons and Ionic Compounds
Ions
Cations and anions can also be expressed through electron configurations. To do so, simply add or subtract the number of electrons that were added or removed from the neutral atom. For example, if atomic number 8 loses 1 electron to become a cation, the electron configuration will reduce by one, and will increase by one if it gains one electron.
🎥Live Stream Replay: Unit 1 Review
Noble Gas Configurations
To further condense things, when the number of electrons gets tedious to write out, it is handy to refer to the nearest noble gas and go from there. Potassium fir example has an electron-configuration of 1s²2s²2p⁶3s²3p⁶4s¹. The nearest noble gas is Argon, with electron-configuration 1s²2s²2p⁶3s²3p⁶. Thus, the electron-configuration of potassium can be shortened to [Ar] 4s¹.
🎮Trivia: AP Chemistry Units 1-2

© 2024 Fiveable Inc. All rights reserved.